Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Development
Degrader progresses in clinical trials for skin conditions
KT-474 improved symptoms of inflammatory skin conditions for 21 people in Phase 1 study
by Bethany Halford
November 20, 2023
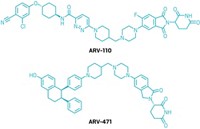
Kymera Therapeutics’ degrader KT-474 has shown promise for treating two inflammatory skin conditions in an early clinical trial. The success suggests that these compounds could be used to treat nonlethal, chronic conditions, where there is a high bar for tolerability.

Degrader molecules, which fight disease by tagging problematic proteins for destruction, have been a hot area of research in recent years. So-called heterobifunctional degraders—also known as proteolysis-targeting chimeras or PROTACs—bind to the troublesome protein at one end and an E3 ligase at the other. This brings the two proteins into close proximity, and the E3 ligase tags the disease-related protein for destruction via the proteasome, known to biochemists as the cell’s garbage disposal.
Although several of these types of degraders are in clinical trials, most are being studied as cancer treatments, where their potential toxicity in a short period of time is acceptable if they can fight the lethal disease. But KT-474 is intended to be given long-term, so it must be tolerable to the people who will take it for years.
KT-474 was designed to degrade the protein interleukin-1 receptor–associated kinase 4 (IRAK4). IRAK4 is a master regulator of innate immunity and is associated with inflammation. Kymera tested KT-474 as an oral treatment for the inflammatory skin diseases atopic dermatitis and hidradenitis suppurativa. The recently published Phase 1 trial demonstrated that KT-474 is safe and tolerable in both healthy volunteers and people with the inflammatory skin conditions. In an open-label portion of the trial with 21 people who have atopic dermatitis or hidradenitis suppurativa, all participants reported that KT-474 improved symptoms, such as itchiness and skin lesions, after 28 days of treatment (Nat. Med. 2023, DOI: 10.1038/s41591-023-02635-7).
Jared A. Gollob, Kymera Therapeutics’ chief medical officer, says the results show these degrader molecules have applications outside of oncology. “There’s a lot of opportunity in immunology, in particular, to really address large populations with oral small molecule degraders that have the power of injectable biologics but have the convenience and safety of oral drugs,” he says.
This is the first time that a peer-reviewed journal has published a clinical trial of a heterobifunctional degrader. Fleur Ferguson, who studies PROTACs at the University of California, San Diego, says the publication is important for this young field. “Being able to see how they’ve approached the small molecule design, seeing the chemical structure, their biochemical characterization, their clinical trial design, and their pharmacokinetics is really exciting,” she says.
Ferguson notes that the trial represents an early read out for the drug candidate and that long-term effects and efficacy remain to be seen. Sanofi is advancing KT-474 in Phase 2 clinical trials, where researchers will study its effectiveness and look for side effects in a larger pool of participants.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter