Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Consumer Safety
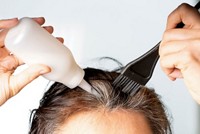
Goodbye to lead in hair dyes
by Britt E. Erickson
October 30, 2018
| A version of this story appeared in
Volume 96, Issue 44

The U.S. Food & Drug Administration has banned the use of lead acetate as a color additive in hair dyes sold in the U.S., according to a final rule published on Oct. 30. The action comes in response to a 2017 petition, filed by public health groups claiming that lead acetate is neurotoxic and carcinogenic.
FDA agreed with the petitioners, stating, “There is no longer a reasonable certainty of no harm from the use of this color additive.”
Many manufacturers have already replaced lead acetate with bismuth citrate in hair dyes applied over time to gradually color hair. But a few products intended to darken gray hair in men still contain lead acetate. FDA will begin enforcing the ban in one year to give companies time to reformulate their products.
“FDA’s decision is an important step to protecting people from a continued source of exposure to lead that is a more significant route than the agency originally thought over three decades ago,” Tom Neltner, chemicals policy director at the Environmental Defense Fund, said in a statement. EDF is one of the groups that petitioned FDA.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter