Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Lab Safety
Nitrate reduction route makes aryldiazonium chemistry safer
Nitrates’ slower reactivity prevents accumulation of explosive diazonium intermediates
by Brianna Barbu
April 30, 2024
As a rule, it’s generally advisable to tread carefully with things that can explode in the laboratory. But it can be difficult to avoid dangerous compounds when the explosive substance is a key player in a number of useful organic transformations—as diazonium salts are.
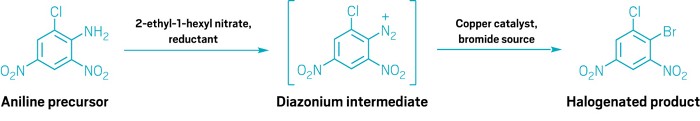
A new approach to taming aryldiazonium chemistry, published in Science by Tobias Ritter and his group at the Max Planck Institute for Kohlenforschung, may help scientists reap the synthetic benefits with less risk (Science 2024, DOI: 10.1126/science.adn7006). Their take on the Sandmeyer reaction, a halogenation method that starts with a diazonium salt, takes advantage of kinetic insights to moderate the buildup of reactive intermediates.
“Everybody knows that they’re dangerous,” says Ritter. “We no longer have to accumulate them.”
Aryldiazonium salts are made from the reaction of an aniline derivative with nitrosonium (NO+). Since the 1800s, the status quo has been to generate NO+from nitrite salts (NO2-) and acid. Ritter and coworkers found that reducing nitrate (NO3-) salts with thiosulfate provides a more gradual release of NO+, which prevents the diazonium from collecting in high concentrations before it proceeds to the halogenation reaction.
Nitrate is relatively slow reacting, which can pose challenges for other applications involving nitrate reduction, such as cleaning up pollution from fertilizer runoff. But in this case, the nitrate reduction’s slower pace was the key to keeping the diazonium chemistry under control. “The relative rates are just right,” says Ritter.
The researchers successfully demonstrated their version of the Sandmeyer method on a selection of medicinally relevant building blocks and several anilines whose diazonium salts have been associated with lab explosions.
The reagents are inexpensive and common in most organic labs, so “everybody can reproduce this experiment,” says Ritter. He says that his team is working on improving and extending the nitrate method to other diazonium reactions as well as thinking about how to scale it up practically given that some of the intermediates are gases.
Craig Merlic, an organic chemist at the University of California, Los Angeles and executive director of the UC Center for Laboratory Safety, says in an email that the paper offers “excellent insights” and a “potentially useful method” for avoiding accumulation of explosive diazonium intermediates. But he says there are still some issues: some of the protocols involve producing gaseous by-products in a closed container, and nitrates can pose their own explosion risks. He recommends that thermochemical studies be done to assess the method’s overall safety before translating it to industrial scale.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter