
HOW CHEMISTRY ROCKS
MUSIC
FESTIVALS
The science enables and enhances the all-encompassing live music experience.
Illustration by Alexander Wells
A festivalgoer flicks through her smartphone. She holds it up and strikes a pose with her friends. She tags it #festival #music, then posts her first group selfie.
The festivalgoer and her friends are among tens of thousands of people who will be captivated by continuous, pulsing beats and dazzled by light-emitting diode (LED) displays. And they'll want to share the experience. Social media sites, such as Instagram, will be awash in photos, videos, stream-of-consciousness thoughts, and hashtags.
And all of it—the music, lights, media, and even the stimulation itself—will be made possible by chemistry.
Giving a guitar its voice
The music festival gets underway as a guitar starts to sing. How do a few kilos of maple wood, strung taut, achieve such a warm, spellbinding sound?
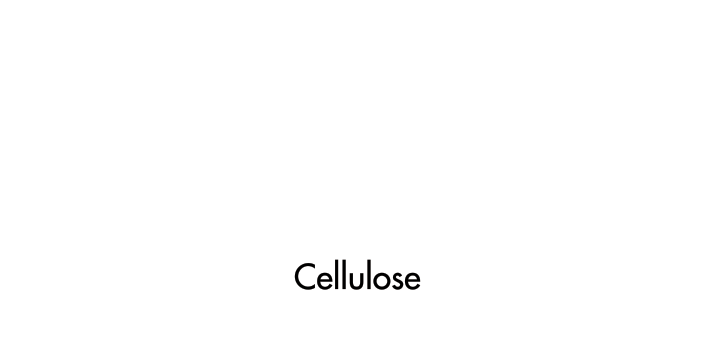
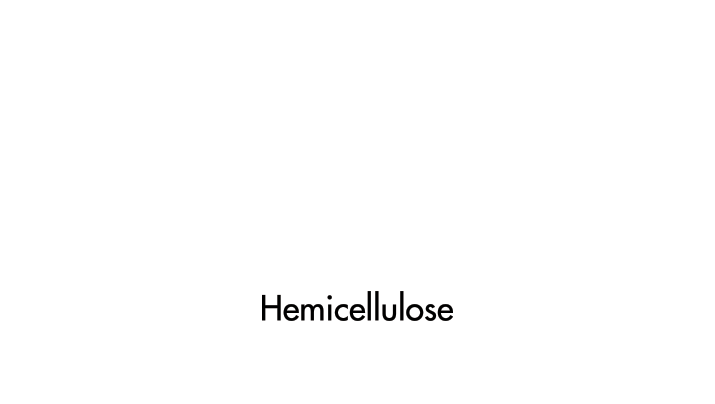
The wood's cellular structure plays a vital role, explains Scott Buehl, a master builder at the Fender Custom Shop, which produces made-to-order musical instruments. Wood is a composite of two polymers, cellulose and hemicellulose, held together by lignin, a complex polymer. The density of the wood, the size of the polymer pores, and other factors affect the resonance of an instrument—for example, by amplifying or dampening sound.
Cellulose and hemicellulose, the polymers composing wood, play an essential role in creating the instrument's distinctive tones.
Wood is not the only factor determining a guitar's voice. Natural and synthetic polymers or alloys in strings, as well as the chemical properties of finishes, such as nitrocellulose, also contribute to the instrument's tone.
The magnetic materials in electric guitar pickups—often alloys of aluminum, nickel, and cobalt—shape the final sound.
A master builder's understanding of the chemistry and structure of materials is key to producing high-quality custom guitars.
Chemical processes can also be used to change the instrument's voice, Buehl explains. “Wood can be treated in a torrefaction process involving heat in the absence of oxygen to enhance its tonal character,” he says. This process removes water and organic volatiles from the wood to produce a richer, warmer tone.
Paints and finishes are also important in determining a guitar's voice. More expensive guitars are finished with nitrocellulose, which goes on thin, Buehl explains. “As it cures, it shrinks further and becomes more brittle. It tends to sound better than our polyester and urethane finishes.” Nitrocellulose lets the wood “breathe” to preserve its natural tones rather than muffle it with cheaper, thicker finishes.

Chemistry also affects the sound of electric guitars. Magnetic pickups consisting of a permanent magnet, or series of magnets wrapped with coil, generate a signal amplified through a speaker as sound. “The sound will vary substantially with the use of various magnetic materials,” Buehl says. Buehl and his team typically use ceramic magnets and three versions of Alnico, which is a group of alloys mostly composed of aluminum, nickel, and cobalt.
Buehl and makers like him rely on their knowledge of the wood, strings, finishes, and metals to create guitars with an array of tones—including mellow, crisp, and lilting.
Music to the ears
Through craft and chemistry, the guitar still produces nothing more than vibrations that pass through air. How do those become something we perceive as music? The answer: chemical reactions and electrical impulses.
The vibrations produced by the guitar and the rest of the band move through the air until they're captured by the pinna, the outer ear. The capture sets off a chain of chemical actions: The sound waves travel down the auditory canal to the eardrum, causing that delicate membrane to vibrate; its movement sets three tiny bones, the ossicles, in motion; this motion in turn transmits the sound into the cochlea—a snail-shaped structure composed of two fluid-filled labyrinths.
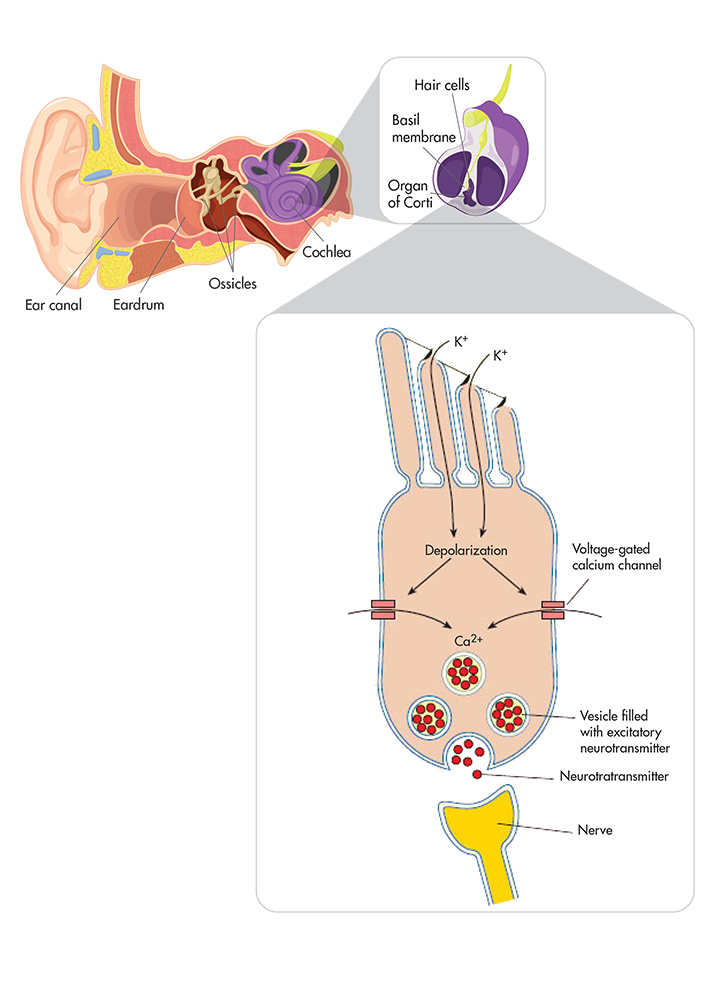
Inside the cochlea, fluids induce vibrations in the basilar membrane, which cause the bending of tiny hairs on the organ of Corti, a receptor and transductor. In the hair cells, chemical processes, such as calcium signaling and potassium-ion depolarization, play a critical role in signal transduction in the inner ear. The chemical processes deliver information about sound acceleration and gravity through the small number of hair-cell mechanosensitive transduction channels to the ribbon synapse and then to the brain. Finally, the vibration of guitar strings become the experience we know as music.
A symphony of emotions
A melody whispers through the crowd, stilling everyone as it reaches their ears. Some feel a chill down their spines, while others get a lump in their throats. These intense physiological responses are activated by chemicals—a process that has long fascinated Valorie N. Salimpoor, a neuroscientific consultant based in Vancouver, British Columbia.
One afternoon, as a graduate student at McGill University's Montreal Neurological Institute, Salimpoor was driving around aimlessly, feeling low and unsure about her life's path. Then Brahms's “Hungarian Dances” came on the radio. “I got chills all over my body,” she recalls. “It was so powerful, I had to pull over, because I really wanted to enjoy that feeling.”
Ever since that day, Salimpoor has been investigating how music affects the brain. “Music is really just a series of sounds organized in time,” Salimpoor says. “Each sound independently has no reward or emotional value for us. But somehow, when these sounds are arranged together in time and patterns, it can do something really magical to our brains.”
Using a positron-emission tomography scanner, Salimpoor identified how music sets off a dopamine release in people's brains, triggering intense emotions (Nat. Neurosci. 2011, DOI: 10.1038/nn.2726). “There are actually two phases of dopamine release in two different regions: one during this peak emotional response, when people experience chills or shivers, and one before, in anticipation of it,” she explains.
The cause is an interaction between dopaminergic regions of the brain and the superior temporal gyrus, the latter being where all the music we've ever heard—and the resulting emotions and memories—are stored as templates.
As we listen, our brains start to follow patterns of sound. Soon we're predicting where the music will go next. This creates anticipation and triggers a rewarding dopamine release for a person disposed to the music. No two superior temporal gyri are the same, though. “It's a very individualized process, because it depends on your specific templates and how much emotional value you've associated with each,” Salimpoor says. “Ultimately, this explains why no two people have the exact same taste in music.”
There is a sweet spot, though: Unfamiliar patterns—such as atonal music—or patterns too predictable trigger less or no dopamine release, Salimpoor explains. That delightful chemical rush that gives us the chills is a delicate dance of familiarity, uncertainty, prediction, and anticipation.
Lighting up with LEDs
By nightfall, the crowd has seen a steady stream of acts enter and exit the stage, but the headliners are still to come. Now a new element latches on to the sensory overload—lighting design. As the first headliner takes the stage, LEDs descend from a scaffold. Shimmering sculptures seem to cross the stage of their own accord as lighting designers run preprogrammed schemes.
Stephen Lieberman's company, SJ Lighting, designs stages for some of the biggest music events around the world. Lieberman is enthusiastic about how LED technology has revolutionized his industry—including video displays, motorized lights, and strobes—and brought a host of benefits, including reduced power consumption and better color rendering.
Pre-LED, reds used to come out an orangey amber, Lieberman says. Now they are purer. “You get a much broader spectrum of color choices, and as designers, that broadens our toolbox and allows us more flexibility and creativity,” he says.
LEDs, otherwise known as light-emitting diodes, emit light from semiconductor materials. These semiconductors often are made of materials, such as gallium nitride and indium phosphide, that have been doped with various elements to make p-type and n-type semiconductors. N-type semiconductors have excess electrons; p-type semiconductors have excess positively charged holes. When an n-type material is bonded to a p-type material, a p-n junction or diode is created; the boundary of the two materials is called the active region.
When an electric current passes through the semiconductors, excess electrons in the n-type material are drawn toward the positive terminal that caps the p-type material. These electrons encounter the holes present in the p-type material. Some of the electrons recombine with holes in the active region through the process of electroluminescence. The process produces a drop from a higher energy level to a lower one and the release of the energy as photons.
The energy difference between the two materials—known as the band gap—plays a role in determining emission colors. Smaller band gaps result in emissions with longer wavelengths, perfect for producing red light; larger band gaps lead to emissions with shorter wavelengths, such as for blue light. The elements in the p- and n-type materials determine the band gap of the diode and, ultimately, the LED colors. Compositions of aluminum gallium indium phosphide produce warm colors, such as red, orange, and yellow; indium gallium nitride compositions emit cooler colors, like blue and green.
Let it glow
The stage may pulse with carefully designed light displays, but the audience has its own warm glow. Glow sticks produce light as chemiluminescence caused by a chemical reaction, most commonly produced by combining hydrogen peroxide; a phenyl oxalate ester, such as diphenyl oxalate; and a fluorescent dye.
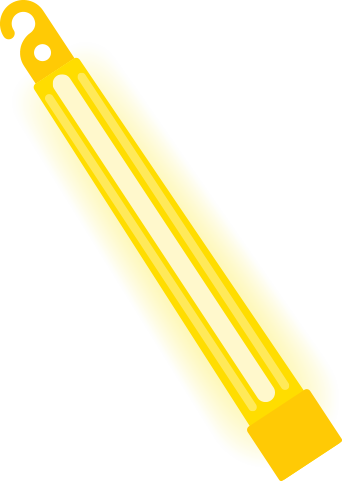



The hydrogen peroxide rests in an inner glass tube that breaks when the glow stick is bent. When released, the hydrogen peroxide reacts with diphenyl oxalate to produce phenol and 1,2-dioxetanedione (peroxyacid ester). The 1,2-dioxetanedione is unstable and decomposes, resulting in a cyclic peroxy compound that in turn breaks down, producing carbon dioxide and energy. Electrons in the fluorescent dye molecules absorb the energy, exciting the dye molecules. As the molecules fall back to their ground states, they release energy in the form of light.
Several innovators have been credited with the invention of glow sticks, including Edwin Chandross, who observed the chemiluminescent phenomenon that makes light sticks glow in 1962 at Bells Labs. In a 2013 Vice story, he said he had no idea they were so popular at music events.
Chemiluminescence continues to fuel cutting-edge innovation across fields as varied as the military and leisure fishing. According to a 2018 study, this glowing substance even has applications for the detection of peroxynitrite, a mediator in the aging process (Free Radical Biol. Med., DOI: 10.1016/j.freeradbiomed.2018.02.017).
Pics or it didn't happen
The festivalgoer's social feeds are now filled with carefree selfies and somewhat blurry photos of bands and food. A crowd as massive as one at a music festival pushes out a staggering number of social media posts. In fact, at Coachella 2017, AT&T customers consumed 29.2 TB of mobile data—equivalent to 83.5 million social media posts with photos, says the company.
The company's high-capacity (and wonderfully named) innovations include the Giant Eyeball Antennae, a spherical cell antenna which emits 18 radio frequency beams, and The Drum Set Antenna—debuted at Coachella in 2017—which offers 30. These innovations are erected on-site using portable units known as cells on wheels (COWS) or cells on light trucks (COLTS), which hold the antennae in place.
These high-capacity antennae are modeled after the Luneburg lens, a spherically symmetric gradient-index lens first developed in the 1940s and now used in cell-phone technology. “A typical Luneburg lens's refractive index n decreases radially from center to outer surface,” explains Bob Mathews, RAN Engineer at AT&T. “They can be made for electromagnetic radiation, from visible light to radio waves.”
To handle a heavy data load, AT&T also uses a distributed antenna system, known as DAS, which helps to boost coverage and capacity. A DAS network consists of many small antennas that enhance wireless service within a specific area, or building, reducing the distance data has to travel.
Temporary technologies can enhance the company's network to help keep the data flowing at events, such as music festivals, where thousands of people gather and use their mobile devices at the same time. “We discovered that the best way to provide strong, consistent cellular coverage to a massive crowd was to divide it into narrow slices and cover each slice separately with its own dedicated antenna beam,” says Mathews.

The company is also looking at how the use of cell and antenna carrying drones, or Flying COWS (cells on wings)—which were first deployed to aid disaster relief in Puerto Rico following Hurricane Maria—might be adapted to keep festivalgoers connected.
Let the music play on
After the last act plays its final encore, the crowd begins to disperse. Exhausted, but content, the festivalgoer and her friends say they've had the best time ever. The festival will linger in their minds long after the afterimage of LEDs fades and typing of hashtags ends. It's the memories of the music, the lights, and the experience that will live on, encoded in festivalgoers' brains by chemistry.
CLARIFICATION: This story was updated on June 7, 2018, to clarify the credit given to Edwin Chandross regarding glow sticks and to cite a missing source. Chandross had observed the chemiluminescent phenomenon that makes light sticks glow in 1962 at Bell Labs but had not created the sticks. He had told Vice in 2013 that he had no idea glow sticks were popular at music events.
Chemours: Precipitating the Conversation
A Perspective from Mark Vergnano, President and CEO of The Chemours Company

At Chemours, we think of ourselves as helping to create a colorful, capable, and cleaner world through the power of chemistry. Our products are critical ingredients in buildings, transportation, electronics, home goods, and energy solutions—all essential to making life better the world over. So why delight? How does a word that evokes carefree fun and even unbridled joy fit our world of discovery, application development, and purposeful, solution-driven commerce? Well, simply like this: Chemistry is also essential to how we have fun.
Anatomy of delight
Think of it this way. All of us who read C&EN are more than just engineers, scientists, and business professionals. We're also musicians, weekend warriors, craftspeople, gourmands, visual artists, and more. We express ourselves—our fun-loving selves—in so many ways that are made possible by those molecules we manipulate, those formulas we pore over, and those experiments we undertake during our day jobs. Chemistry makes fun possible!
I rarely stop to wonder at the car that I drive to work or the insulation that keeps my house comfortable, but exciting and unusual experiences do stand out for me—like the opening ceremonies of the PyeongChang Winter Olympics. The drones, augmented reality, and pyrotechnics amazed me—so much so that I asked myself, how'd they do that? The answer, of course, is chemistry.
With that in mind, in this series we've set out to take a closer look at the chemistry underlying other magical, memorable experiences we share with our friends and families, aiming to highlight the role chemistry plays in these moments of exuberance and wonder. Our products that go into building materials, communications, electronics, truck and car manufacturing, mining, energy storage and generation, coatings, and refrigeration help bring these experiences to life, just as they enable the routine but necessary.
Dimensions of delight
Delight is serious business to us, and we take pride in our contribution to helping bring treasured pastimes and glee to people around the globe. But we're even prouder of our relentless focus on another dimension of delight—delighting our customers. Chemours brings a relentless customer-first focus to our suite of trusted, iconic brands, combining best-performing chemical compounds with our preoccupation with meeting the needs of our customers and the markets they serve. To us, delighting our customers may mean co-designing a new formulation or anticipating a new application the market will need in years to come. It may mean getting ahead of large-scale technological shifts so that when our customers require special capabilities or properties from our chemistry, we're ready with just the right product.
At Chemours, we call this being customer centered—one of the values that animates our company. It drives us forward to find new applications for proven chemistry and to find new chemistry to make life better and, yes, sometimes even fill us with delight.
I hope you enjoy this series. And perhaps the next time you go to a concert, have a great meal, ride a roller coaster with your kids, or spend a day in the great outdoors, you'll think about chemistry, our shared passion—because, ladies and gentlemen, what we do makes all of this possible.
READ ABOUT HOW CHEMOURS HELPS ENABLE DIGITAL AUDIO:
July 30: The Chemistry of Outdoor Recreation
August 20: The Chemistry of Amusement Parks
October 1: The Chemistry of Sports
November 26: The Chemistry of Travel
December 17: The Chemistry of Dining
ABOUT SPONSORED CONTENT
Sponsored content is not written by and does not necessarily reflect the views of C&EN's editorial staff. It is authored by writers approved by the C&EN BrandLab and held to C&EN's editorial standards, with the intent of providing valuable information to C&EN readers. This sponsored content feature has been produced with funding support from The Chemours Company.








