Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Atoms swap spots in aromatic rings
New reaction replaces carbon with nitrogen
by Bethany Halford
October 12, 2023
| A version of this story appeared in
Volume 101, Issue 34
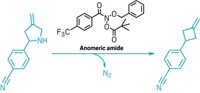
Replacing a carbon atom in an aromatic ring with a nitrogen atom is one of those exercises that’s easy to do on paper with a pencil and eraser but tough to accomplish in a reaction flask. Chemists have now developed a reaction that does precisely that. They start with an aromatic azide and replace the carbon the azide is attached to with a nitrogen.
The new reaction gives medicinal chemists another skeletal editing transformation they can use to modify drug candidates. Putting a nitrogen in place of carbon in these molecules’ aromatic rings can result in dramatic improvements in key pharmacological parameters—so much so that medicinal chemists have dubbed it the “necessary nitrogen atom.”

To determine if there’s a necessary nitrogen atom, chemists will perform nitrogen scanning, in which they sequentially replace carbon atoms in an aromatic ring with nitrogen to see how those changes alter a pharmaceutical candidate’s properties. Nitrogen scanning is typically done by building each molecule from scratch. University of Chicago chemistry professor Mark D. Levin, who led the research effort, says this new reaction, when combined with C–H activation methods, could make nitrogen scanning much easier.

“It’s a reaction that prior to this nobody thought was possible,” says Tyler J. Pearson, the report’s first author and a postdoctoral scholar in Levin’s group.
The chemists start with an aromatic azide and use a photochemical reaction with ethylaminoethanol, followed by oxidation withN -bromocaprolactam. Only the carbon that was attached to the azide is replaced by nitrogen, making the swap intuitive, Levin says. The researchers used the transformation to make an azasteroid derivative of estrone (shown), which had been made previously via a longer route from less-available starting materials (Science 2023, DOI: 10.1126/science.adj5331).
“As part of a lecture I gave on the necessary nitrogen atom, I said this skeletal editing reaction is science fiction today but may be science fact in 20 years,” says Lewis Pennington, a consultant with Mystic River Medicinal Chemistry. “That was 3 years ago.”
“When we dream about one-pot chemical transformations for drug discovery, swapping carbon into nitrogen is near the top of the list,” David Rees, chief scientific officer at Astex Pharmaceuticals, says in an email. The new reaction from Levin’s group, he says, “is just about as good as we’ve seen so far.” He points out that for the atom swap to be widely used in the pharmaceutical industry, there are still questions about scale-up, azide handling, and functional group tolerance.
Pennington points out that there are limitations to the reaction. A chemist would need the ability to do this reaction predictably and selectively on every single one of a molecule’s aromatic carbon atoms, which isn’t possible yet. “This is a baby step, but it’s a huge baby step. It’s like your baby’s first step,” he says.
Levin agrees. “It is a huge breakthrough, but it doesn’t solve every problem in that space,” he says. Chemists need to be able to install the azide onto the aromatic ring, and the transformation doesn’t work with every azide because of competing reactions. He sees this version as a first-generation reaction with upgrades to come. “This is a reaction that deserves to be made even better,” he says.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter