Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
Chemistry In Pictures
Chemistry in Pictures: Messy plate
by Manny I. Fox Morone
October 14, 2021

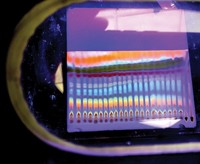
These bulbous bands of color are what was left after Maria-Elena Liosi tried to purify the product of her reaction on a preparatory thin-layer chromatography (TLC) plate. Like the smaller, more familiar TLC plates, this square plate is coated with a bit of silica which wicks solvent from the shallow pool at the bottom of a glass chamber. As the solvent carries a reaction mixture deposited on the bottom of the plate upward through the silica, it separates the various molecules in the mixture based on their polarity, creating these bands which can scraped off the plate and isolated.
Despite the appeal of the groovy upper band on this plate, Liosi, a PhD student studying synthetic medicinal chemistry in the lab of William L. Jorgensen at Yale University, found that both the desired product of her reaction and a by-product were mingled together at the top of the plate. So she had to use other methods to complete the separation.
Submitted by Maria-Elena Liosi. Follow Liosi @chemistrystories on Instagram.
Do science. Take pictures. Win money. Enter our photo contest here.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter