Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Medicinal Chemistry
Slow and steady action makes brain cancer drug candidate more selective
Researchers designed a molecule that kills brain cancer cells while giving healthy cells time to repair their DNA
by Brianna Barbu
June 1, 2024

Glioblastoma is one of the most common, and deadly, forms of brain cancer. Only about 7% of people with glioblastoma will survive more than 5 years after being diagnosed according to the National Brain Tumor Society. Many glioblastoma tumors lack a DNA-repair enzyme called O 6-methylguanine methyltransferase, or MGMT, which reverses unwanted DNA alkylation.

Researchers from Yale University, led by organic chemist Seth Herzon and oncologist Ranjit S. Bindra, used their understanding of organic leaving-group kinetics to design a molecule that deals fatal DNA damage to cancer cells while minimizing collateral harm to healthy cells. “It all comes down to basic chemistry,” Herzon says.
The standard chemotherapy for glioblastoma is temozolomide, which disrupts tumor cells’ DNA by sticking methyl groups to guanine bases. Without functioning MGMT, cancer cells can’t repair the methylated guanines, and the bases mispair, leading to cell death. But tumors often evolve resistance to temozolomide.
Lomustine, which doctors may then turn to, also works by alkylating guanine. But rather than adding a methyl group to a guanine, lomustine attaches a 2-chloroethyl group. This group quickly cyclizes to create an ethanoguanine intermediate. The ethanoguanine reacts with a cytidine base in the complementary DNA strand, creating interstrand cross-links that block the DNA from replicating, leading to cell death in the tumor. But ethanoguanine can also harm healthy cells.

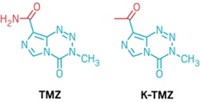
The researchers wanted to give MGMT in healthy cells time to remove the unwanted alkyl groups while still having those groups cause fatal DNA cross-linking in MGMT-deficient cancer cells. They reasoned that replacing the chlorine from lomustine’s chloroethyl group with a more reluctant leaving group, such as fluorine, would buy that time by slowing down the cyclization reaction that forms ethanoguanine.
After evaluating several temozolomide derivatives with varied cyclizable alkyl groups, the researchers landed on KL-50, which contains a fluoroethyl group. They reported the compound, the rationale behind its design, and studies showing that it shrinks tumors in mice in a 2022 Science paper (DOI: 10.1126/science.abn7570).
Now, in the Journal of the American Chemical Society, the researchers flesh out how KL-50 works and how the leaving-group kinetics affect its selectivity for cancer cells (2024, DOI: 10.1021/jacs.3c06483). In long-term cell survival assays and a screening panel of 902 human cancer cell lines, the molecule selectively killed MGMT-deficient cells.
The researchers found that synthesized DNA duplexes containing fluoroethyl guanine started showing signs of cross-linking in about 5 h when MGMT was absent; the duplexes showed little sign of cross-linking when MGMT was there. This key experiment illustrated how KL-50 spares healthy cells while still damaging MGMT-deficient cancer cells. Strikingly, DNA duplexes made with chloroethyl guanine rapidly formed cross-links with or without MGMT around and also reacted with MGMT to form DNA-protein linkages, suggesting two ways that lomustine can harm healthy cells.
Kent Gates, a chemist at the University of Missouri who researches DNA-damaging natural products and small molecules that fight tumors, praised the work as “a careful and comprehensive study of the chemical and biochemical mechanisms” that make KL-50 effective. He says the added insight into DNA-protein cross-linking is “particularly clever and insightful.”
Herzon, Bindra, and colleagues cofounded Modifi Bio, in 2021 to help advance KL-50 toward clinical trials. They hope to finish preclinical safety studies within a year or so and start testing in humans sometime in 2025. Herzon says that being able to work on research that might help people with glioblastoma live longer, fuller lives has been incredibly rewarding to him as a chemist. “It’s an opportunity to take our basic knowledge in fundamental physical organic chemistry and apply it in a way that’s meaningful,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter