Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biocatalysis
Enzyme and photocatalyst team up to make nonnatural amino acids
The bond-forming power of radical chemistry meets the stereoselectivity of biocatalysis
by Brianna Barbu
July 27, 2023
| A version of this story appeared in
Volume 101, Issue 25
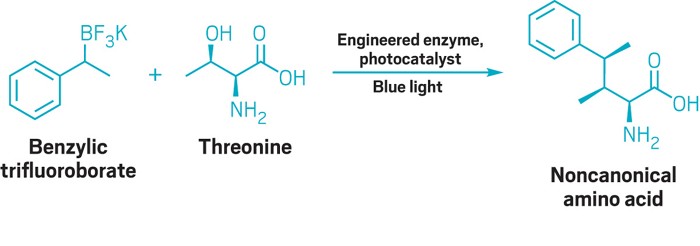
It is a truth universally acknowledged in chemical synthesis that a molecule in posession of multiple stereocenters must be difficult to make. But the combined abilities of biochemistry and radical chemistry could help make stereoselective amino acid synthesis a lot less troublesome.
Yang Yang and his team at the University of California, Santa Barbara, found that pairing an engineered enzyme with a radical-generating photocatalyst could stitch together noncanonical amino acids that have up to three adjacent stereocenters (Science 2023, DOI: 10.1126/science.adg2420).
“We’re merging the best things from the two worlds” of biochemistry and sythetic chemistry, Yang says.
Noncanonical amino acids are valuable building blocks for designer proteins and peptide drugs. Making them stereoselectively using traditional synthesis methods requires multiple tedious protection and deprotection steps. Two adjacent stereocenters can be set with some difficulty, Yang says, but any more than that is practically unheard of in synthetic chemistry.
Combining radicals and enzymes could open up synthetic possibilities previously unknown to either chemistry or biology, Yang says. Radicals are very handy for forging carbon-carbon bonds, but they aren’t traditionally stereoselective. Enzymes, meanwhile, are experts at constructing molecules with precise 3D geometry in ways that ordinary synthetic methods can’t access. They can also be engineered to enhance their abilities.
Yang and his team directed their enzyme-engineering efforts toward a family of enzymes that use pyridoxal 5′-phosphate (PLP) as a cofactor. PLP-dependent enzymes are specialists at modifying amino acids through a well-known mechanism that goes through an aminoacrylate intermediate. The researchers thought they would be great for intercepting and incorporating a radical. They created two variants of the PLP-dependent tryptophan synthase β subunit—one version optimized for producing D-amino acids and the other for L-amino acids.
The researchers use a rhodamine photocatalyst to generate carbon-centered radicals from benzylic trifluoroborate salts. The radicals then make their way into the active sites of the enzymes, which stick them onto the β position of an amino acid to create substituted tryptophan analogs—no protecting group needed. The reaction also works for some nonbenzylic radicals, though the yields were much lower—the alkyl radicals are so short lived it was exciting to see that they worked at all, Yang says.
The dual-catalyst system successfully modified serine, which has one stereocenter, and threonine, which has two stereocenters, with high enantioselectivity and diastereoselectivity. Using a racemic trifluoroborate created a prochiral radical that, when attached to threonine, resulted in a molecule with three adjacent stereocenters.
Todd Hyster, whose research group at Princeton University also works on merging photocatalysis and enzymes, says the work has “all the hallmarks of a really great biocatalysis paper” because it uses simple starting materials and efficiently accomplishes a reaction that would take many steps to do otherwise. He and others have taken a similar tack with other enzyme families, but this is the first example he’s seen of radical biocatalysis with PLP enzymes. There are many different PLP enzymes that catalyze a variety of reactions, so this sort of dual-catalyst approach could open up many other types of synthetically useful reactions, he says.
Yang says he and his team will keep working on engineering their PLP enzymes to be more efficient, finding variants that select for other stereoisomers, and continuing to expand the toolbox of radical enzymes for assembling noncanonical amino acids. “There’s definitely a lot more interesting enzyme functions to be discovered,” he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter