Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
C-H Activation
Technique turns C–H neighbors into diamines
Combo of electro- and photochemistry allows double C–H activation
by Leigh Krietsch Boerner
February 4, 2021
| A version of this story appeared in
Volume 99, Issue 5

Researchers have upped the ante on carbon-hydrogen bond activation by transforming two adjacent C–H bonds in one reaction. Tristan Lambert and Tao Shen at Cornell University used a mix of light and electrochemistry to convert neighboring C–H bonds on simple hydrocarbons to cyclic amine and diamine derivatives, molecules often found in useful drug compounds (Science, 2021, DOI: 10.1126/science.abf2798). This rare double activation method offers a new path to synthesizing valued molecules and a relatively simple way to create powerful catalysts.
Very few double C–H functionalizations are known, mainly because activating one C–H bond tends to make the surrounding C–H bonds less reactive. The key was turning on the trisaminocyclopropenium ion catalyst first through electrochemical oxidation, then with white light, Lambert says. This oxidation-first approach makes the catalyst energetic enough to activate both C–H neighbors but allowed the researchers to apply a current below the threshold that would oxidize the ethane reactants. “This gives you the potency of this massively oxidizing, excited state intermediate,” Lambert says.
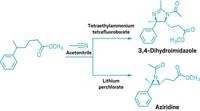
The team can also control its products by changing the electrolyte. In lithium perchlorate, the compound adds an acetonitrile from solution, then reacts internally to form a cyclic aziridine compound (shown, bottom). If the electrolyte is tetrabutylammonium fluoride, the ethane adds first one, then a second acetonitrile to form a dihydroimidazole (shown, top). With its strategy, the team was able to synthesize over 65 compounds, including a variety of drug-molecule analogues. This suggests that the technique is useful for late-stage functionalization, a way to add functional groups to existing molecules instead of making them from scratch.
The reaction does a 4-electron oxidation of the ethane, which shows the true power of using electrochemistry, says David Nicewicz, a photochemist at the University of North Carolina at Chapel Hill. If the team had used a chemical oxidant, “they would need a truckload,” he says. “In this case, they just need a current.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter