Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Light-activated catalyst makes syngas greener
System highly stable, has high selectivity
by Leigh Krietsch Boerner
January 17, 2020
| A version of this story appeared in
Volume 98, Issue 3

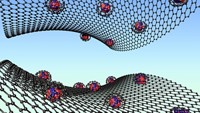
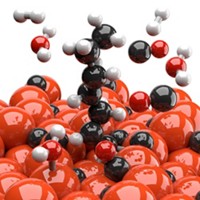
Synthesis gas, or syngas, is a feedstock for multiple industrial processes, including the Haber-Bosch process for making ammonia. Syngas is a mixture of carbon monoxide, carbon dioxide, and hydrogen. Gasification plants, which make syngas from coal, natural gas, and other sources, use catalysts to break apart fuels at around 800 °C. Naomi Halas and coworkers at Rice University have now come up with a greener way to make syngas (Nat. Energy 2020, DOI: 10.1038/s41560-019-0517-9). Using light to activate a nanoparticle catalyst made of copper antennas and single ruthenium atom reactor sites, the team was able to make syngas at around 200 °C. Their system is stable up to 50 h with 99% selectivity. Current industrial syngas catalysts are less robust because they’re susceptible to coking, a C–C bond-forming process that leads to the growth of a carbonaceous material that can block active sites and poison the catalyst. “By having isolated reactive sites, they’re not next door to another active site, so you can’t make C–C bonds very easily,” Halas says. The group has licensed the technology to Syzygy Plasmonics, a company Halas cofounded, to adapt the technology for industry. This could provide a greener path to syngas, which in turn could reduce carbon emissions and energy use in many industrial processes.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter