Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Light-driven reaction selectively flips aldehyde isomers
Reaction converts chiral aldehyde mix into a single isomer
by Leigh Krietsch Boerner
March 3, 2022
| A version of this story appeared in
Volume 100, Issue 9

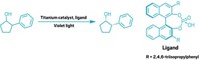
Converting a mixture of mirror-image forms, or stereoisomers, of a molecule into a single isomer is a big deal in making pharmaceuticals, because usually only one isomer is useful as a drug. But current options for this process, called deracemization, are complicated and fiddly. Research by Sanzhong Luo and coworkers at Tsinghua University and the Haihe Laboratory of Sustainable Chemical Transformations has now made it as simple as turning on a light. The group used an iridium compound, an enamine, and violet light to transform a mixture of branched aldehyde isomers into a single one (Science 2022, DOI: 10.1126/science.abl4922).
The work combines two well-understood reactions: double-bond photoisomerization and enamine catalysis, which was recognized with the 2021 Nobel Prize in Chemistry. The group’s straightforward approach can be used to deracemize mixtures of many types of chiral aldehydes.
The method works by transferring the chirality, or handed-ness, from the target molecule onto a photoswitchable chiral catalyst. After light converts this catalyst to the desired isomer, another reaction transfers the new chirality back to the original molecule. Starting with an equal mix of chiral right- and left-handed aldehydes (R- and S-, respectively), Luo and coworkers added an amine that only reacts with the S-aldehyde, exclusively forming one enamine isomer. The team hit the solution with 400 nm light, flipping the enamine to its opposite isomer. The enamine isomer then splits to form the desired R-aldehyde. The enamine acts as a shuttle between R- and S- interconversion, Luo says. To show proof of concept, the group synthesized several nonsteroidal anti-inflammatory (NSAID) compounds, including ibuprofen and loxoprofen.
“This is an elegant solution to an important and very challenging problem,” Alison Wendlandt, a catalytic chemist at the Massachusetts Institute of Technology, says in an email. “There is little doubt in my mind that this tool will be adopted by other chemists and that the authors’ approach will inspire additional development in this area. Very cool!”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter