Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Nickel catalyst enables versatile amine synthesis
Method creates hundreds of different amines from handy nitriles
by Mark Peplow, special to C&EN
June 29, 2022
| A version of this story appeared in
Volume 100, Issue 24


Amines are ubiquitous in the pharmaceutical and chemical industries, and a nickel-based catalyst has now opened up a promising route to make these molecules from widely-available nitrile compounds (Science 2022, DOI: 10.1126/science.abn7565).
Primary amines, in which a nitrogen atom is bonded to just one carbon, are often made by reducing nitrile groups using hydrogen. It’s an efficient and cost-effective approach, widely used in the production of nylon, surfactants, and plastics. In contrast, the various methods used to make secondary or tertiary amines, where nitrogen carries two or three carbon groups, tend to be more complicated and involve more waste.
So chemists have been searching for ways to use nitriles to make secondary and tertiary amines using a process called hydrogenative coupling. This involves reacting a nitrile with hydrogen to produce a primary imine intermediate, and then coupling the imine to a primary or secondary amine. This coupling displaces the imine’s nitrogen in the form of ammonia, and forges a new C–N bond to produce a secondary or tertiary amine.
The problem is that route generally produces a mess of different products. One reason is that the imine intermediate can itself react with hydrogen to make an unwanted amine, which then muscles in on the coupling reactions.
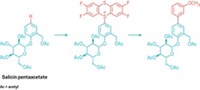
A team led by Rajenahally Jagadeesh and Matthias Beller at the Leibniz Institute for Catalysis has now developed a catalyst that can steer the hydrogenative coupling reaction along the right course, ensuring high selectivity for the desired product. The key is to make sure that the reaction between the imine intermediate and hydrogen is much slower than the intended coupling reaction, which minimizes unwanted side reactions.
After testing a range of different catalysts and conditions, the researchers settled on a champion catalyst that contain nickel bound to a triphosphine ligand. The reaction worked well at 100–120 °C and 4000 kPa of hydrogen, and the team used this process to make more than 230 different amines. “We can make complex agrochemicals and pharmaceuticals, and it also has a very wide applicability for the fine- and bulk-chemical industries,” says Jagadeesh.
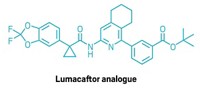
The catalogue of compounds made with the reaction featured many chiral molecules, including the drug tecalcet, used to treat conditions related to the parathyroid glands. The method can also couple nitriles with ammonia, an approach the researchers used to make primary amines labeled with a nitrogen-15 isotope that could be used in metabolic studies. The team prepared a handful of products at the 10 g scale, and Jagadeesh says the reaction should be amenable to further scale-up.
“This research is excellent. I was overwhelmed to see how many amines could be prepared,” says Yasunari Monguchi at Daiichi University of Pharmacy. One particular advantage, Monguchi says, is that it uses a very simple catalyst system that relies on a commercially available ligand.
The reaction worked well with precursors containing a range of different functional groups, although it stumbled when faced with aldehydes, alkynes, and a few other groups. This versatility means the procedure could be used to modify nitrile-containing compounds at the end of a long synthesis—a strategy known as late-stage functionalization—to make many analogs of drug candidates for high-throughput screening, for example.
The team is now investigating the mechanism of the reaction and hopes to reduce the temperature and pressure of the reaction using a cobalt-based catalyst that is showing early promise.
CORRECTION:
This story was updated on June 30, 2022, to correct Yasunari Monguchi's title. Monguchi is a full professor, not an assistant professor.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter