Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Phosphines that act like metal catalysts
Inexpensive trialkylphosphines can catalyze C–F activation
by Brianna Barbu
January 29, 2024
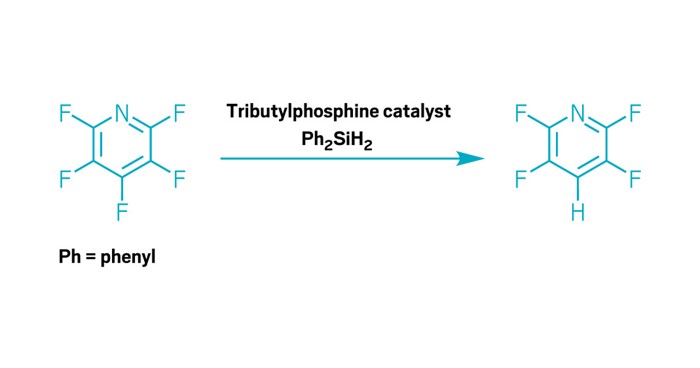
Phosphines have earned a promotion. A new paper shows that phosphines, long known for supporting transition metals in catalytic reactions, are worthy of a starring role. Researchers from the University of Toulouse and the University of York have demonstrated that commercially available tributylphosphine can act as a catalyst to break carbon-fluorine bonds on organic molecules and swap in hydrogen or an amine—no metal needed (J. Am. Chem. Soc. 2024, DOI: 10.1021/jacs.3c10614).
“Redox cycling in main-group chemistry has been this holy grail for a really long time,” says John Slattery of the University of York, one of the research team’s leaders. Phosphorus can happily sit in either the +3 or +5 oxidation state, and it’s not difficult to get it to switch between them. The challenge is to get it to do that in a catalytic cycle.

This isn’t the first time anyone has accomplished that particular feat. A group from Tel Aviv University published a phosphine-catalyzed C–F activation last year (J. Am. Chem. Soc. 2023, DOI: 10.1021/jacs.2c13318). But the catalyst in that research was a bespoke pincer-shaped compound; this new work shows that the fancy structure isn’t needed for a phosphine to act as a catalyst.
Antoine Simonneau of the University of Toulouse says the project started when graduate student Sara Bonfante saw some unexpected side products in a C–F activation reaction with a zirconium complex, which hinted that the phosphine ligand was doing its own reaction. So she decided to look into that reactivity further. Investigating the mechanism of what was happening enabled the rest of the pieces to fall into place, Simonneau says.
The steps that the phosphorus goes through in the reaction are the same as what a typical metal catalyst does. First is oxidative addition to break the C–F bond. Then the phosphine passes the fluoride to a silane in exchange for a hydride or amide. Finally, reductive elimination regenerates the catalyst and creates the final product.
Catherine Weetman of the University of Strathclyde says in an email that while main-group catalysis remains rare and challenging, this paper “should make everyone take note of the potential power of main group elements” for cost-effective chemistry.
Slattery says he can’t reveal too many details about the next steps he and his collaborators have planned, but he says they are working on optimizing and extending phosphines’ reactivity for practical applications. He hopes that these results will continue to inspire research into main-group chemistry. “This is certainly the beginning of the story of these elements working in this way.”



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter