Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
A greener alternative to Wittig
Reaction ditches ylides for a recyclable Pd catalyst
by Leigh Krietsch Boerner
January 10, 2020
| A version of this story appeared in
Volume 98, Issue 2

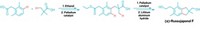
The Wittig reaction, in which chemists use phosphorus ylides to make alkenes out of aldehydes or ketones, is both well known and vastly important to industry. Generating ylides requires hazardous or toxic reagents such as n-butyllithium, however, as well as multiple solvent-thirsty purification steps. Adelina Voutchkova-Kostal and coworkers at George Washington University have now developed a greener alternative to the Wittig reaction by using a recyclable palladium catalyst (J. Am. Chem. Soc. 2019, DOI: 10.1021/jacs.9b12354). The researchers synthesized and tested multiple catalysts. The most effective one was based on a Pd-alumina catalyst and can flip through nine cycles before it begins to lose selectivity. Carbon monoxide and water are the only waste. This reaction shines when making alkenes from aliphatic aldehydes and when combining aliphatic and aromatic aldehydes, Voutchkova-Kostal says. What’s striking about the chemistry is its simplicity, she adds. “It’s combining two reactions that have been around for a long time.” The reaction performs a tandem aldol reaction, which brings the aldehyde and alcohol together, then a decarbonylation step, which spits out CO (shown). The researchers put the two together to make that C–C bond, Voutchkova-Kostal says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter