Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Medicinal Chemistry
Chemists bring substituted bicyclopentanes into reach
Synthetic route developed by Scripps and Pfizer could widen access to arene analogs
by Leigh Krietsch Boerner
November 9, 2020

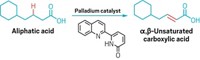
Flat is generally out of favor with medicinal chemists. For several years, drug hunters have been leaning away from substituted planar arenes and toward more architecturally complex compounds, which often have better solubility, permeability and stability and sometimes better bioactivity. Now Phil Baran, Michael Collins, and coworkers at Scripps Research and Pfizer have developed a new synthetic route for a class of non-planar molecules on medicinal chemists’ wish list, but were previously hard to access in high yields (ChemRxiv 2020, DOI: 10.26434/chemrxiv.13120283.v1). The work has yet to undergo peer review.
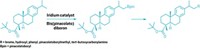
Chemists have long known that 1,3-difunctionalized bicyclopentanes can act as bioisosteres, compounds that have different structures but similar drug properties, for para-substituted arenes. Researchers have suspected that ortho- and meta-substituted bicyclopentanes could also act as bioisosteres but lacked the data to support their theory. That’s because they’re more challenging to make in large quantities—in fact, Baran’s group tried several different strategies before landing on one that worked. The successful synthesis involves first making a bicyclopentane precursor, then using that compound to make several different kinds of ortho- and meta-substituted benzene analogues (shown).
The team is in the process of testing the compounds through both biological and computational studies to see if they are suitable bioisosteres. If they are, the bicyclopentanes might get around limitations of the substituted arene rings, which can be metabolized into potentially toxic compounds.
These difunctionalized bicyclopentanes have a similar 3-D shape as the arene, but the body can’t metabolize them in the same problematic way as the arene, Baran says. “Now you have the same orientation in an enzyme pocket, but without the problem of the liver chewing it up and turning into something toxic.”
This is one of the few—and one of the strongest—examples of syntheses that allows scientists to mimic the meta- and ortho- isomers, says Daniele Leonori, an organic chemist at the University of Manchester. Medicinal chemists in industry will be very excited about this development, he says.


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter