Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
This nitrene has staying power
Most nitrenes exist for mere nanoseconds; this one hangs around for days
by Bethany Halford
June 13, 2024
| A version of this story appeared in
Volume 102, Issue 18

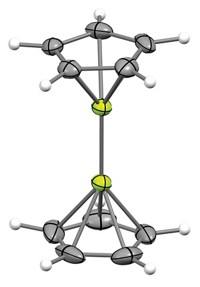
Nitrenes—octet-defying molecules with a neutral nitrogen atom bonded to only one other substituent—are typically so reactive that their lifetimes are measured in nanoseconds. But now chemists have created a nitrene that lasts for 3 days. The molecule is likely to find use as a ligand for transition-metal complexes, much like carbenes did in the 1990s, say University of Bremen chemists Jens Beckmann and Emanuel Hupf, who led the work.
The new nitrene owes its long lifetime to its surrounding substituent: a scaffold known as MSFluind, which was first reported in 2011 by Kohei Tamao of Kyoto University and colleagues. In recent years, chemists have used variations of MSFluind to stabilize other fleeting intermediates, including a bismuthinidene and a stibinidene.
This bulky scaffold prevents the nitrene from reacting and provides electronic stabilization. “With this magic substituent, we’re basically building up a wall behind the nitrogen atom,” Beckmann says.
Beckmann and Hupf’s team crystallized the nitrene and studied it using X-ray crystallography. Because the molecule is a ground-state triplet, meaning it has two unpaired electrons, the researchers also used electron paramagnetic resonance spectroscopy and superconducting quantum interference device magnetometry to characterize it (Science 2024, DOI: 10.1126/science.adp4963).
Guy Bertrand, who studies octet-defying molecules at the University of California San Diego, says it’s impressive that the Bremen team was able to isolate the triplet nitrene. He says in an email that the work also shows that the “MSFluind family of substituents will allow for the isolation of hitherto elusive species, not only in main group chemistry, but also in transition metal chemistry.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter