Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Synthesis
The rules for assembling azetidines
Researchers devise criteria for cycloaddition between acyclic oximes and alkenes
by Brianna Barbu
June 28, 2024
| A version of this story appeared in
Volume 102, Issue 20
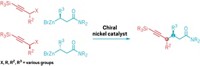
Azetidines, four-sided rings made of three carbon atoms and a nitrogen, are a handy molecular motif in applications as diverse as drug design and rocket fuels. The most efficient way to assemble them is via the aza Paternò–Büchi reaction, a light-powered cycloaddition between an alkene and an imine. But because the imine has a tendency to isomerize when it’s photoexcited, the reaction has historically been limited to imines that are locked into a cyclic structure.

Now a collaboration led by Corinna Schindler of the University of Michigan and Heather Kulik of the Massachusetts Institute of Technology has figured out the rules for joining acyclic imines and activated alkenes into never-before-seen azetidine structures (Science 2024, DOI: 10.1126/science.adj6771). “It’s a big step forward for us,” Schindler says.
Using a combination of experimentation and computational modeling, the researchers determined three key factors for success: The frontier orbital energy of the imine must match that of the alkene after it’s been activated by the photocatalyst, which is why oxime esters work well as imine components. The transition state energy has to be lower than the energy barrier for alkene dimerization. And the oxime carbon must have enough accessible surface area.
“There’s a lot of different reactions you can rule in or rule out before you ever do the experiment,” Kulik says.
Tehshik Yoon, who researches photocatalytic organic transformations at the University of Wisconsin–Madison and was not involved in the work, says this paper is “a really valuable contribution” to the art of making azetidines.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter