Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
ACS Meeting News
Microfluidics could enable rapid at-home testing for common STIs
A device similar to home COVID-19 tests could provide highly sensitive detction of 3 common bacterial infections
by Max Barnhart
August 20, 2024

The World Health Organization reports that around the world, people acquire more than a million curable sexually transmitted infections (STIs) every day. Most of those STIs, however, are asymptomatic. That fact should motivate people to obtain screenings routinely. But stigma associated with talking to one’s doctor about STIs or going to a sexual health clinic means that many folks never get screened and could be living with one or more undiagnosed infections.
At the American Chemical Society Fall 2024 meeting in a talk in the Division of Biological Chemistry, Elijah Barstis, a chemistry PhD student in Chuck Henry’s research group at Colorado State University, presented a new, inexpensive microfluidics-based device for detecting bacterial STIs, including chlamydia, gonorrhea, and syphilis. Barstis said the device is based on a modified enzyme-linked immunosorbent assay (ELISAs) and that the user steps are similar to those for a home COVID-19 test. “You take a sample, dip it in a buffer, add a couple drops into the device itself, and then after 15 to 20 min, you’ll have a visual readout,” he said. The devices can also be combined into a single unit, allowing for simultaneous detection of multiple STIs.
Barstis explained that the seven-layered device works through capillary action and contains no moving parts. As the sample flows through channels in the device, it rehydrates several reagents, goes through a wash step, and follows a process similar to what’s typically performed in a lab setting. The reagents include an antibody specific to a particular STI and an enzyme label that reacts with the substrate to produce a colorimetric readout.
That enzyme’s reaction with the chromogenic substrate is what allows the device to be highly sensitive. Barstis said currently available laboratory ELISA tests for chlamydia can detect proteins on the surface of the cell at a concentration somewhere between 5 and 100 ng/mL of sample. The data show that this microfluidic device is comparably sensitive, detecting chlamydia at protein concentrations as low as 5.27 ng/mL.
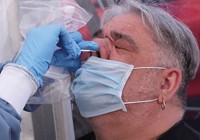
But one potential barrier to using this STI testing platform is that it requires the user to perform an uncomfortable and somewhat invasive genital swab instead of producing a urine sample. Henry said urine tests for STIs can be prone to false negatives.
James Hougland, a biochemist at Syracuse University who wasn’t involved in the presented research, said that the data presented at the meeting were “really nice validation” that an ELISA-like assay could be performed in a microfluidics-based device, and he sees this technology enabling more people to take control of their health. “I could totally see this being something that would be [available to take home from] university health centers,” he said.
This STI testing device is just one of several microfluidics-based testing devices that Henry’s group is working on. Other tests the group is developing include a COVID-19 test and a test for detecting biomarkers of heart failure, which was also presented at ACS Fall 2024. Henry hopes that the simplicity of using these microfluidic devices, along with how inexpensive they are compared with lab tests, will help get more people checked for these easily treatable STIs.
That effort will take years, Henry said. His company Burst Diagnostics is actively developing these microfluidics-based tests for commercialization, but he says that more work is needed to refine this technology before the devices can proceed to trials for US Food and Drug Administration approval.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter