Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
While metals play indispensable roles in our world as the frames of vehicles, the skeletons of buildings, and the moving parts of machinery, they also have a weakness: corrosion. Oxidants in the environment can slowly weaken and degrade metals.
Vitals
Current affiliation: University of Western Ontario
Age: 30
PhD alma mater: McGill University
Hometown: Cambridge, Ontario
My role model is: "Marie Curie because of her resilience in the face of adversity, her dedication to education, and her commitment to scientific integrity."
My favorite movie is: "My Big Fat Greek Wedding because it shares a core heartwarming message about the importance of love, acceptance, and embracing one’s identity."
Samantha M. Gateman wants to help these crucial materials stand up to corrosion. The University of Western Ontario (UWO) chemist studies corrosion down to the scale of individual atoms to better understand, predict, and prevent it. Her work could help safely store spent nuclear fuel for millennia, extend the lifetimes of modern cars and trucks, and even improve birth control.
While metals like stainless steel might appear uniform to the eye, they look very different at the atomic level. “Under a microscope you can see grains and inclusions and precipitates that make the microstructure heterogeneous,” Gateman says. Corrosion can start at those regions that differ from the bulk metal. Identifying vulnerable spots can help manufacturers learn how to produce metals that are more resistant to corrosion. For example, if Gateman discovers corrosion in areas that are richer in one element than the surrounding metal, she can suggest finding a new alloy or manufacturing technique that would reduce those heterogeneities.
These microscopic vulnerabilities might not matter much for your silverware, but it’s a different story for a metal canister holding nuclear fuel.
Canada’s Nuclear Waste Management Organization (NWMO) is the government agency tasked with safely storing spent fuel from the country’s nuclear power plants. The NWMO plans to seal used fuel pellets inside steel and copper capsules and then bury them hundreds of meters deep in clay and rock. The fuel will remain radioactive for thousands of years. If the capsules corrode, they could leak and contaminate groundwater—and radioactive material could eventually reach rivers or lakes.
This is where Gateman comes in. The NWMO is her biggest industrial funder. She uses electron microscopes and other tools to study the capsule metals while they sit in different solutions that simulate what the containers might experience underground. Her team looks for corrosion points and brings that information to the capsules’ designers and manufacturers.
The project requires Gateman to work closely across different fields, a talent that has become one of her trademarks. She collaborates not only with capsule engineers but also with scientists who understand the chemistry of decaying nuclear fuel and the geochemical and microbial conditions that the capsules will experience. Gateman collects all these data to accurately simulate possible corrosion.
Advertisement
“No one does science by themselves,” says electrochemist Janine Mauzeroll, who was Gateman’s graduate adviser at McGill University. “Her ability is to find key people and get the knowledge and put it together.”
In addition to Gateman’s work for the NWMO, she’s studying corrosion of advanced high-strength steels. Car and truck manufacturers are exploring these new alloys because they’re lighter than traditional steel but just as strong, which could lead to higher fuel efficiency. But there is evidence that these alloys are more susceptible to corrosion. That could result in vehicles that require expensive repairs and have a shorter lifespan. Gateman hopes her microscopic investigative procedures can help find fixes.
Another corrosion project Gateman works on is more personal. She is looking for alternatives to the copper-based intrauterine device (IUD), the only long-term, reversible, nonhormonal birth control available. IUDs work through corrosion. Chemical reactions release copper ions from IUDs into the uterus, where the ions disrupt fertilization. Copper IUDs also inflame the uterus and, in some people, cause spotting and heavier periods.
“I asked, as a scientist and a woman, Why copper?” Gateman says. “I wasn’t satisfied with so many negative side effects. I asked if there were other materials we can use.” Zinc and iron ore are possible alternatives she’s investigating. She’s working with researchers at the UWO medical school, as well as with polymer chemists, sociologists, and psychologists, to develop—and maybe eventually market—a new IUD.
Gateman’s various projects focus on the behavior of small collections of atoms, a long way from human experience. But she hopes that the potential outcomes of her work are much more tangible and have a positive effect on people’s lives.
Materials
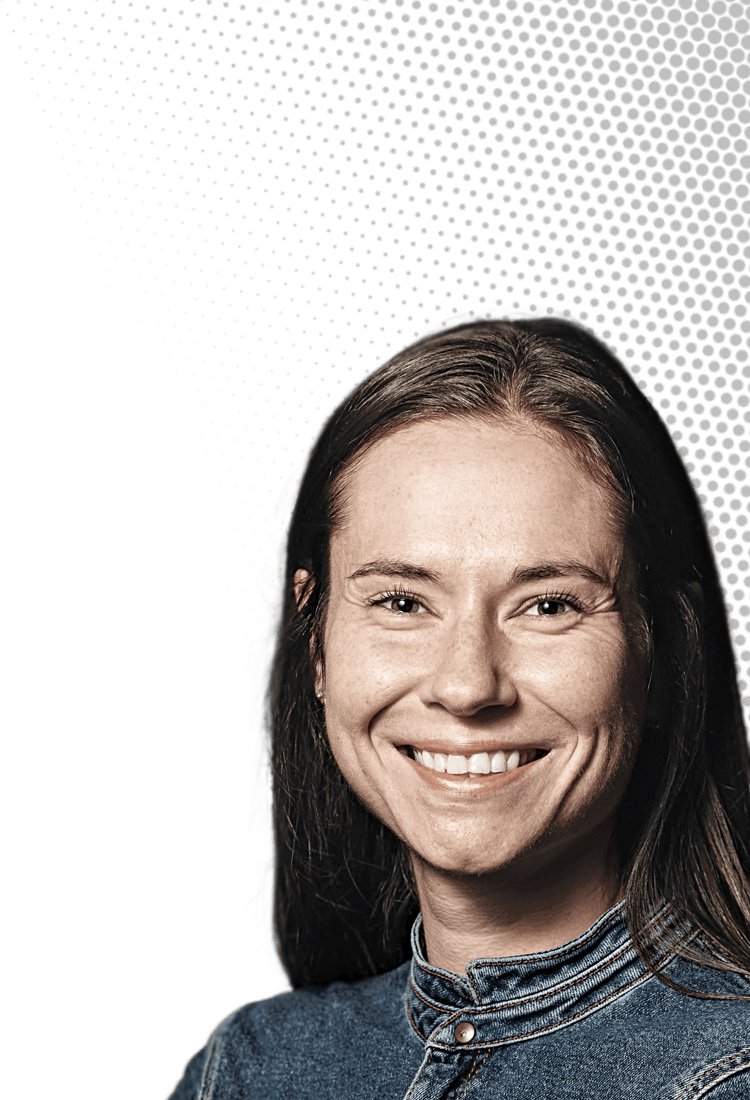
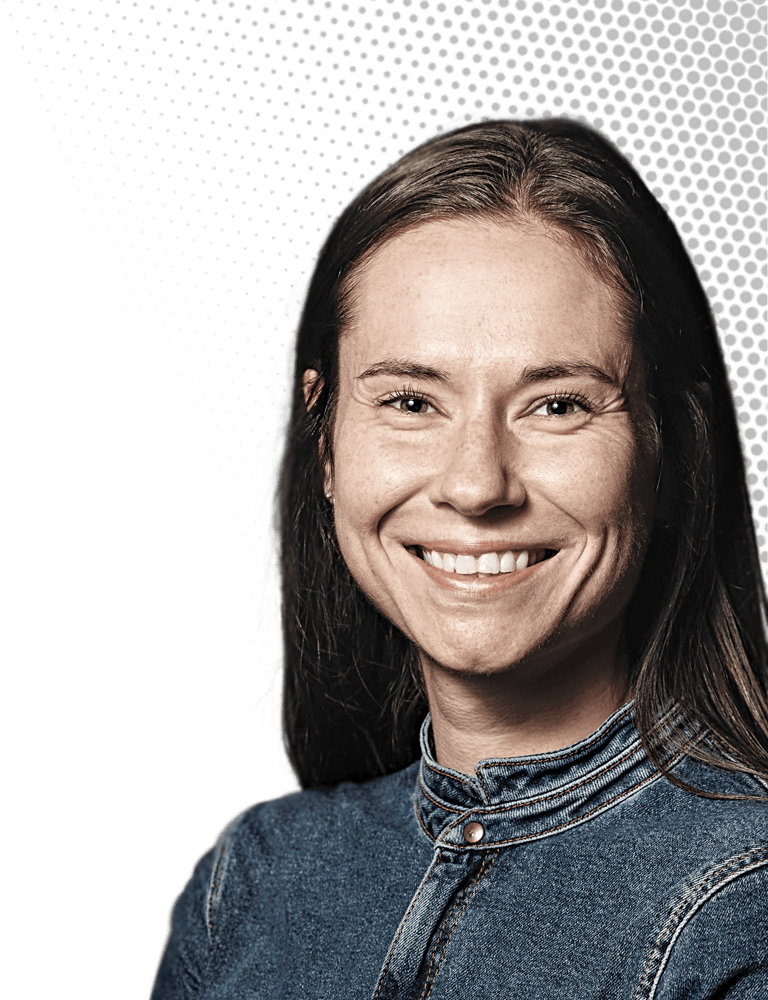
Samantha M. Gateman
This analytical chemist studies metals at the atomic level to prevent corrosion
by Sam Lemonick, special to C&EN
May 17, 2024
| A version of this story appeared in
Volume 102, Issue 15

Credit: Courtesy of Samantha M. Gateman/C&EN | Samantha M. Gateman
Vitals
Current affiliation: University of Western Ontario
Age: 30
MD-PhD alma mater: McGill University
Hometown: Cambridge, Ontario
My role model is: "Marie Curie because of her resilience in the face of adversity, her dedication to education, and her commitment to scientific integrity."
My favorite movie is: "My Big Fat Greek Wedding because it shares a core heartwarming message about the importance of love, acceptance, and embracing one’s identity."
While metals play indispensable roles in our world as the frames of vehicles, the skeletons of buildings, and the moving parts of machinery, they also have a weakness: corrosion. Oxidants in the environment can slowly weaken and degrade metals.
Samantha M. Gateman wants to help these crucial materials stand up to corrosion. The University of Western Ontario (UWO) chemist studies corrosion down to the scale of individual atoms to better understand, predict, and prevent it. Her work could help safely store spent nuclear fuel for millennia, extend the lifetimes of modern cars and trucks, and even improve birth control.
While metals like stainless steel might appear uniform to the eye, they look very different at the atomic level. “Under a microscope you can see grains and inclusions and precipitates that make the microstructure heterogeneous,” Gateman says. Corrosion can start at those regions that differ from the bulk metal. Identifying vulnerable spots can help manufacturers learn how to produce metals that are more resistant to corrosion. For example, if Gateman discovers corrosion in areas that are richer in one element than the surrounding metal, she can suggest finding a new alloy or manufacturing technique that would reduce those heterogeneities.
These microscopic vulnerabilities might not matter much for your silverware, but it’s a different story for a metal canister holding nuclear fuel.
Canada’s Nuclear Waste Management Organization (NWMO) is the government agency tasked with safely storing spent fuel from the country’s nuclear power plants. The NWMO plans to seal used fuel pellets inside steel and copper capsules and then bury them hundreds of meters deep in clay and rock. The fuel will remain radioactive for thousands of years. If the capsules corrode, they could leak and contaminate groundwater—and radioactive material could eventually reach rivers or lakes.
This is where Gateman comes in. The NWMO is her biggest industrial funder. She uses electron microscopes and other tools to study the capsule metals while they sit in different solutions that simulate what the containers might experience underground. Her team looks for corrosion points and brings that information to the capsules’ designers and manufacturers.
The project requires Gateman to work closely across different fields, a talent that has become one of her trademarks. She collaborates not only with capsule engineers but also with scientists who understand the chemistry of decaying nuclear fuel and the geochemical and microbial conditions that the capsules will experience. Gateman collects all these data to accurately simulate possible corrosion.
“No one does science by themselves,” says electrochemist Janine Mauzeroll, who was Gateman’s graduate adviser at McGill University. “Her ability is to find key people and get the knowledge and put it together.”
In addition to Gateman’s work for the NWMO, she’s studying corrosion of advanced high-strength steels. Car and truck manufacturers are exploring these new alloys because they’re lighter than traditional steel but just as strong, which could lead to higher fuel efficiency. But there is evidence that these alloys are more susceptible to corrosion. That could result in vehicles that require expensive repairs and have a shorter lifespan. Gateman hopes her microscopic investigative procedures can help find fixes.
Another corrosion project Gateman works on is more personal. She is looking for alternatives to the copper-based intrauterine device (IUD), the only long-term, reversible, nonhormonal birth control available. IUDs work through corrosion. Chemical reactions release copper ions from IUDs into the uterus, where the ions disrupt fertilization. Copper IUDs also inflame the uterus and, in some people, cause spotting and heavier periods.
“I asked, as a scientist and a woman, Why copper?” Gateman says. “I wasn’t satisfied with so many negative side effects. I asked if there were other materials we can use.” Zinc and iron ore are possible alternatives she’s investigating. She’s working with researchers at the UWO medical school, as well as with polymer chemists, sociologists, and psychologists, to develop—and maybe eventually market—a new IUD.
Gateman’s various projects focus on the behavior of small collections of atoms, a long way from human experience. But she hopes that the potential outcomes of her work are much more tangible and have a positive effect on people’s lives.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter