Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biological Chemistry
Carbohydrate Size Control
Tethering mechanism regulates length of sugar chains for tuberculosis bacterial cell wall
by Stuart A. Borman
July 6, 2009
| A version of this story appeared in
Volume 87, Issue 27
By studying how an enzyme in tuberculosis (TB) bacteria makes uniform-sized sugar chains for the bacteria’s cell walls, researchers have identified a new target for anti-TB medications and have also uncovered a molecular mechanism that enzymes use to control the length of biosynthesized carbohydrates (Proc. Natl. Acad. Sci. USA, DOI: 10.1073/pnas.0901407106).
Carbohydrate oligomers and polymers—including cellulose, a component of plant cell walls, and hyaluronan, an extracellular matrix material in joints and neural tissues—play many essential roles in biological systems. For these compounds to perform their biological functions, their chain lengths must fall within set ranges.
But unlike nucleic acids and proteins, carbohydrate oligomers and polymers are biosynthesized without templates that specify their length and sequence. They are instead made by enzymes that add carbohydrate monomers successively to acceptor substrates, and researchers have not known how these enzymes control chain length.
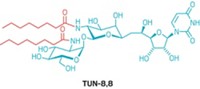
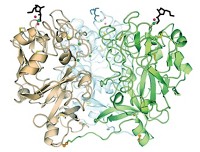
To address this question, chemistry and biochemistry professor Laura L. Kiessling of the University of Wisconsin, Madison, and coworkers investigated the glycosyltransferase GlfT2, which catalyzes the synthesis of galactan, an essential cell-wall polysaccharide in Mycobacterium tuberculosis, the species that causes TB.
They find that GlfT2 controls the length of the polysaccharide by a tethering mechanism. Sugar monomers add to a lipid acceptor at the enzyme’s elongation site. As the polymer grows, it remains attached to the elongation site, and its lead end links to a secondary site. But, as the chain lengthens, the enzyme’s hold becomes increasingly tenuous, and the chain eventually falls away at one of the two sites, causing elongation to stop.
Kiessling and coworkers believe the two-site mechanism may not be restricted to GlfT2. Other glycosyltransferases may use it as well but may have different detachment sensitivities, leading to oligosaccharides or polysaccharides of different prescribed lengths.
“I’ve not seen anything along this line anywhere else in our field, so it really is a new model,” comments Chris Whitfield of the University of Guelph, in Ontario. “It’s going to give the field a bit of a headshake, and we’ll be looking for similar binding sites in other elongating polymerases.”
“As more structural data on GlfT2 and other polymerizing glycosyltransferases become available, it will be interesting to determine the mechanism’s generality,” says Todd L. Lowary of the University of Alberta, Edmonton.
The findings may lead to a new approach for developing TB therapeutics. “The results suggest that targeting both GlfT2 binding sites would generate potent inhibitors of galactan polymerization and cell-wall biogenesis,” Kiessling says. The deeper understanding of biosynthesis that the study affords could also aid design of carbohydrate drugs and production of plant carbohydrate-based fuels, she notes.





Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter