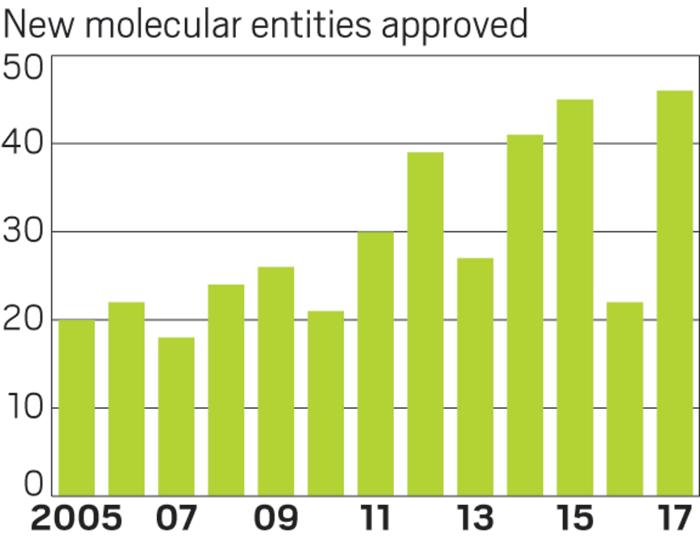
Early on in 2017, it was clear the year would bring a bountiful crop of new medicines. By mid-June, FDA had given its nod to as many new molecules as were approved in all of 2016.
The prior year’s meager output meant many big companies were in need of a rebound in productivity. And indeed, for some, 2017 was a salve. AstraZeneca, for example, had no approvals in 2016. It now has three new products in its portfolio: two cancer treatments and an asthma drug.
Bountiful year
Cancer and rare-disease drugs scored the most approvals in 2017, and small molecules continued to be an important drug modality.
Download a PDF table of the new drugs shown in the slideshow below.

Trulance (Peptide)

Credit: Synergy Pharmaceuticals
Active ingredient: Plecanatide
Applicant: Synergy Pharmaceuticals
Indication: Chronic idiopathic constipation
Mechanism of action Guanylate cyclase-C agonist
Wholesale acquisition cost*: $353/month
Parsabiv (Peptide)
Credit: Amgen
Active ingredient: Etelcalcetide
Applicant: Amgen
Indication: Secondary hyperparathyroidism in chronic kidney disease
Mechanism of action Calcium-sensing receptor modulator
Wholesale acquisition cost*: N/A
Emflaza (Small Molecule)
Active ingredient: Deflazacort
Applicant: Marathon Pharmaceuticals
Indication: Duchenne muscular dystrophy
Mechanism of action Corticosteroid prodrug
Wholesale acquisition cost*: $35,000/year (lowered from $89,000)
Incentive: Rare pediatric priority review voucher
Siliq (Antibody)

Credit: Valeant Pharmaceuticals
Active ingredient: Brodalumab
Applicant: Valeant Pharmaceuticals
Indication: Psoriasis
Mechanism of action IL-17RA antagonist
Wholesale acquisition cost*: $3,500/month
Xermelo (Small Molecule)
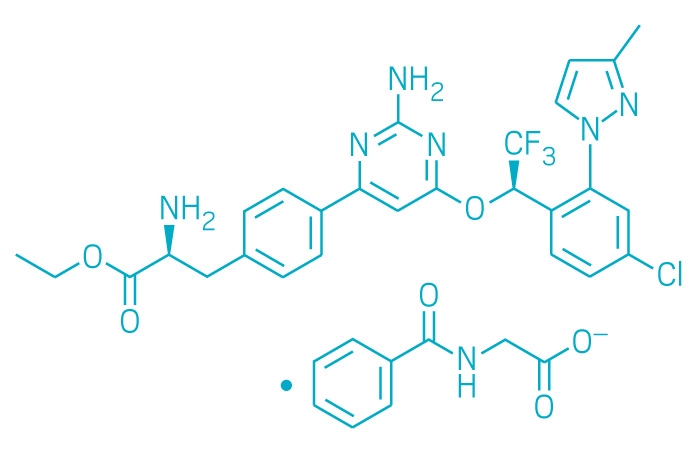
Active ingredient: Telotristat ethyl
Applicant: Lexicon Pharmaceuticals
Indication: Carcinoid syndrome diarrhea
Mechanism of action Tryptophan hydroxylase inhibitor
Wholesale acquisition cost*: $5,164/month
Kisqali (Small Molecule)
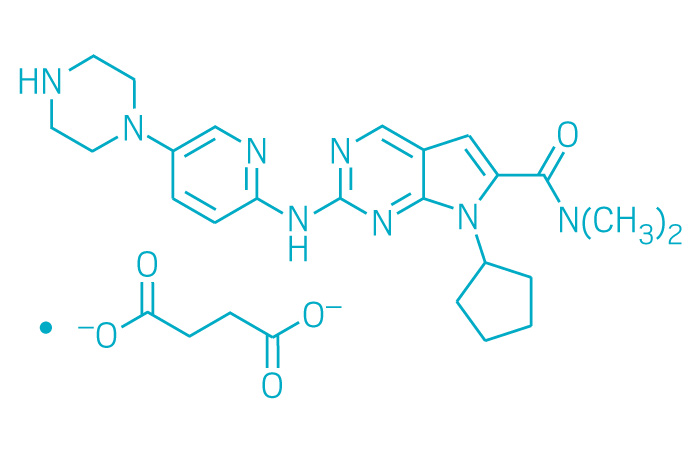
Active ingredient: Ribociclib
Applicant: Novartis
Indication: HR-positive/HER2-negative advanced or metastatic breast cancer
Mechanism of action CDK4/6 inhibitor
Wholesale acquisition cost*: 28-day supply of 600 mg pills is $10,950; 400 mg is $8,760; and 200 mg is $4,380
FDA special status: Breakthrough
Xadago (Small Molecule)
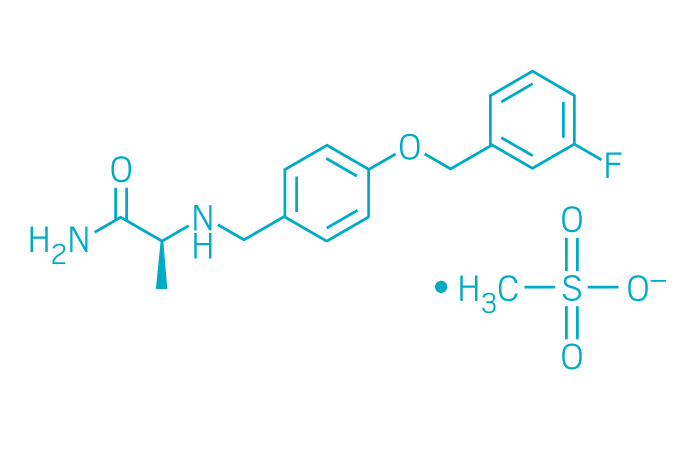
Active ingredient: Safinamide
Applicant: Newron Pharmaceuticals
Indication: Parkinson's disease
Mechanism of action Monoamine oxidase B inhibitor
Wholesale acquisition cost*: $670/month
Bavencio (Antibody)
Credit: Merck KGaA/Pfizer
Active ingredient: Avelumab
Applicant: Merck KGaA
Indication: Merkel cell carcinoma
Mechanism of action PD-L1 inhibitor
Wholesale acquisition cost*: $13,000/month
FDA special status: Breakthrough| Accelerated approval
Symproic (Small Molecule)
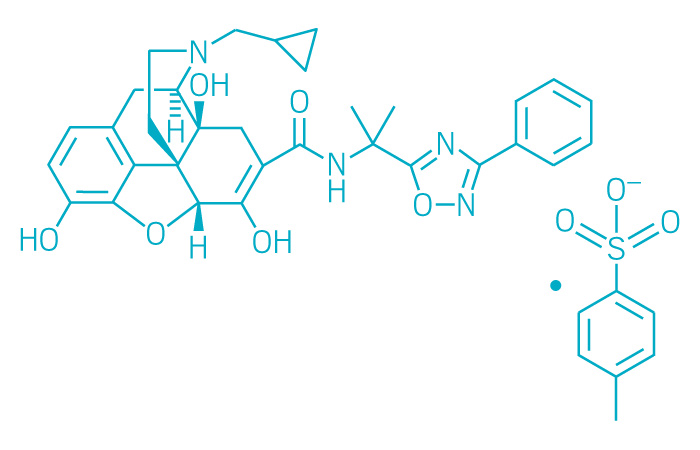
Active ingredient: Naldemedine
Applicant: Purdue Pharma
Indication: Opioid-induced constipation
Mechanism of action Mu opioid receptor antagonist
Wholesale acquisition cost*: N/A
Zejula (Small Molecule)
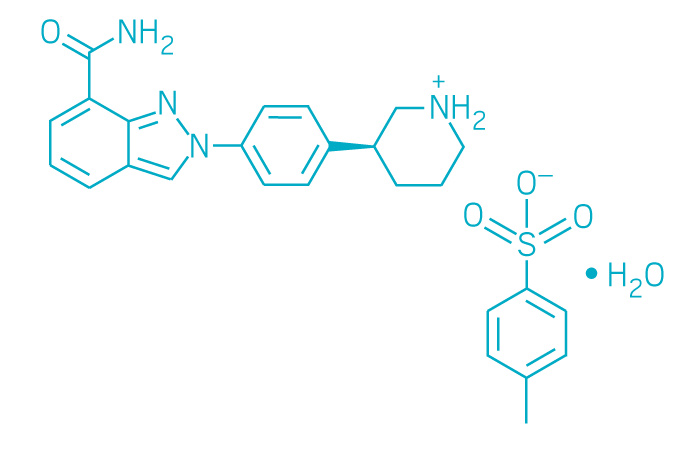
Active ingredient: Niraparib
Applicant: Tesaro
Indication: Recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer
Mechanism of action PARP inhibitor
Wholesale acquisition cost*: $9,833/month for 200 mg/day (recommended 300 mg/day dose)
FDA special status: Breakthrough
Dupixent (Antibody)
Credit: Sanofi/Regeneron Pharmaceuticals
Active ingredient: Dupilumab
Applicant: Sanofi/Regeneron Pharmaceuticals
Indication: Eczema
Mechanism of action IL-4 and IL-13 inhibitor
Wholesale acquisition cost*: $37,000/year
FDA special status: Breakthrough
Ocrevus (Antibody)
Credit: Roche
Active ingredient: Ocrelizumab
Applicant: Roche
Indication: Multiple sclerosis
Mechanism of action CD20 binder
Wholesale acquisition cost*: $65,000/year
FDA special status: Breakthrough
Austedo (Small Molecule)
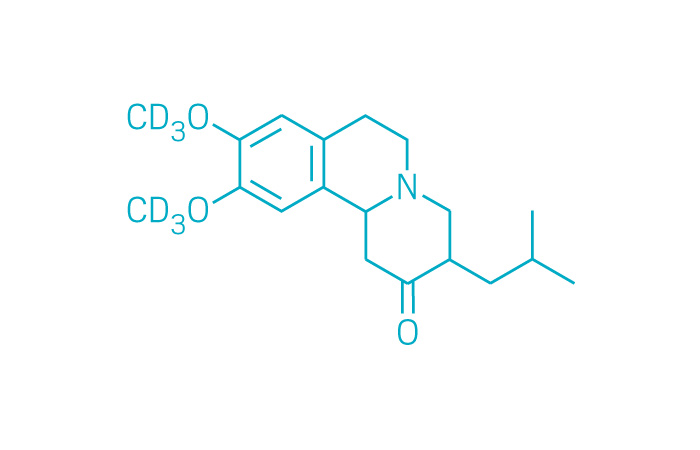
Active ingredient: Deutetrabenazine
Applicant: Teva Pharmaceutical
Indication: Huntington's disease-associated chorea
Mechanism of action Vesicular monoamine transporter 2 inhibitor
Wholesale acquisition cost*: $60,000/year
Ingrezza (Small Molecule)
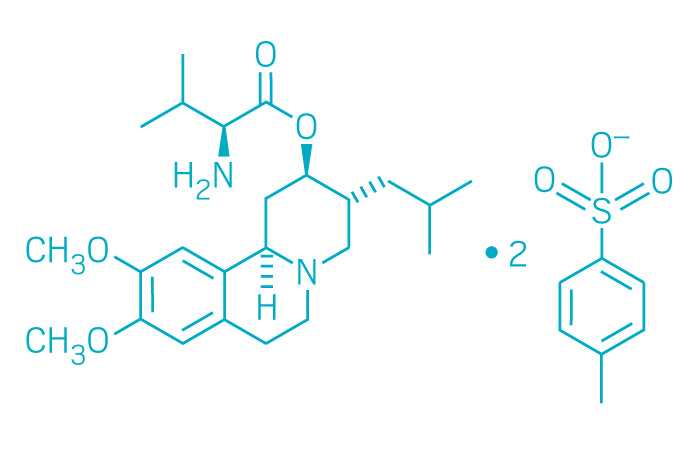
Active ingredient: Valbenazine
Applicant: Neurocrine Biosciences
Indication: Tardive dyskinesia
Mechanism of action Vesicular monoamine transporter 2 inhibitor
Wholesale acquisition cost*: $5,275/month
FDA special status: Breakthrough
Brineura (Enzyme)

Credit: BioMarin Pharmaceutical
Active ingredient: Cerliponase alfa
Applicant: Biomarin Pharmaceutical
Indication: Batten disease
Mechanism of action Enzyme replacement therapy
Wholesale acquisition cost*: $702,000/year
FDA special status: Breakthrough
Incentive: Rare pediatric priority review voucher, sold for $125 million
Alunbrig (Small Molecule)
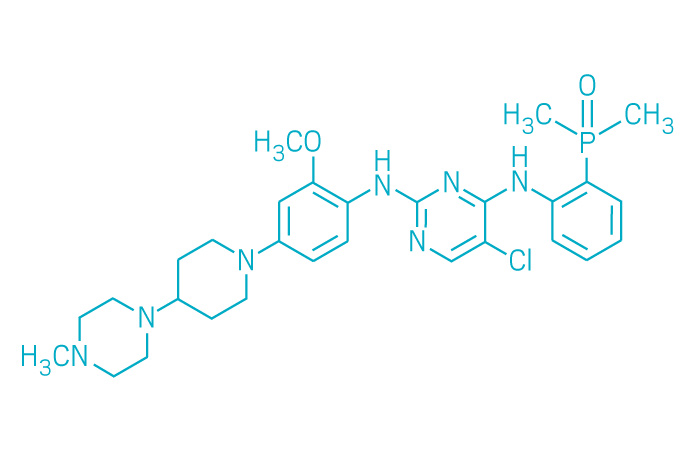
Active ingredient: Brigatinib
Applicant: Takeda Pharmaceutical
Indication: ALK-positive non-small cell lung cancer
Mechanism of action ALK inhibitor
Wholesale acquisition cost*: $14,250/month
FDA special status: Breakthrough | Accelerated approval
Rydapt (Small Molecule)
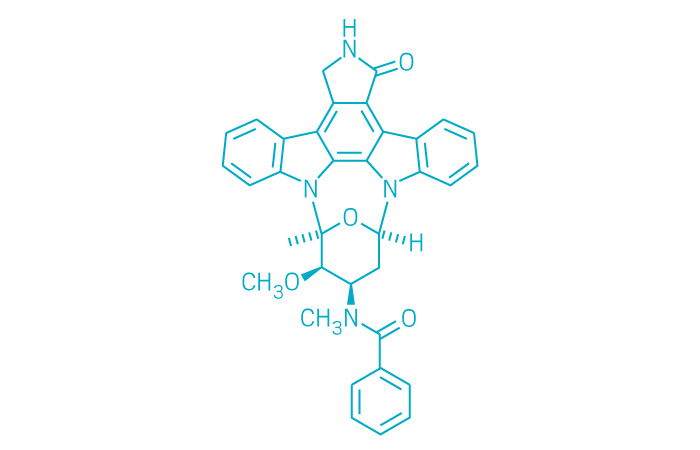
Active ingredient: Midostaurin
Applicant: Novartis
Indication: FLT3-positive acute myeloid leukemia
Mechanism of action Multikinase inhibitor, including FLT3 and KIT
Wholesale acquisition cost*: $14,990/28 days
FDA special status: Breakthrough
Tymlos (Peptide)
Credit: Radius Health
Active ingredient: Abaloparatide
Applicant: Radius Health
Indication: Osteoporosis
Mechanism of action Parathyroid hormone-related protein
Wholesale acquisition cost*: $19,500/year
Imfinzi (Antibody)

Credit: AstraZeneca
Active ingredient: Durvalumab
Applicant: AstraZeneca
Indication: Urothelial carcinoma
Mechanism of action PD-L1 inhibitor
Wholesale acquisition cost*: $180,000/year
FDA special status: Breakthrough | Accelerated approval
Radicava (Small Molecule)
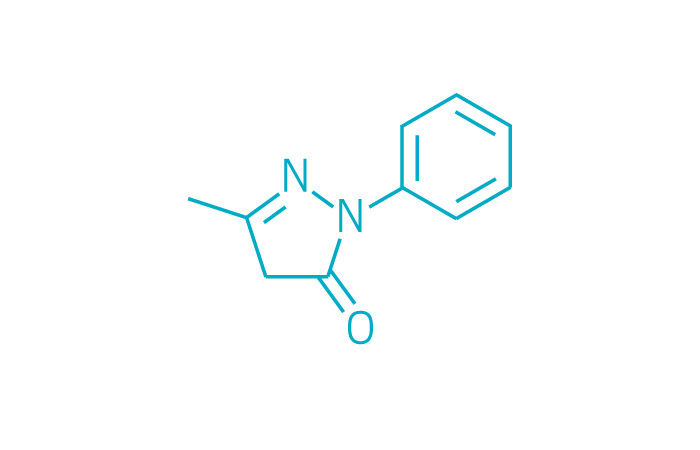
Active ingredient: Edaravone
Applicant: Mitsubishi Tanabe
Indication: Amyotrophic lateral sclerosis
Mechanism of action Unknown
Wholesale acquisition cost*: $145,524/year
Kevzara (Antibody)

Credit: Sanofi/Regeneron Pharmaceuticals
Active ingredient: Sarilumab
Applicant: Sanofi/Regeneron Pharmaceuticals
Indication: Rheumatoid arthritis
Mechanism of action IL-6R inhibitor
Wholesale acquisition cost*: $39,000/year
Baxdela (Small Molecule)
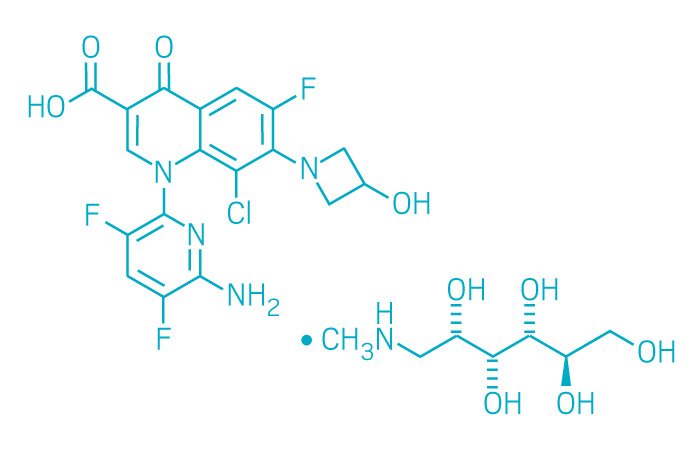
Active ingredient: Delafloxacin
Applicant: Melinta Therapeutics
Indication: Skin infections
Mechanism of action Fluoroquinolone
Wholesale acquisition cost*: N/A
Bevyxxa (Small Molecule)
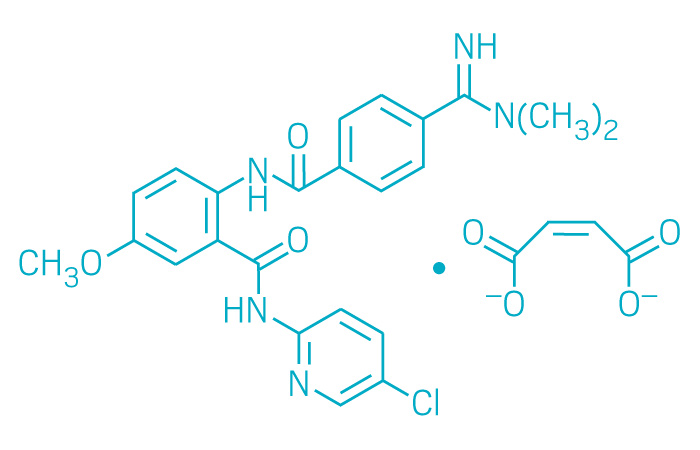
Active ingredient: Betrixaban
Applicant: Portola Pharmaceuticals
Indication: Venous thromboembolism
Mechanism of action Factor Xa inhibitor
Wholesale acquisition cost*: N/A
Tremfya (Antibody)
Credit: Johnson & Johnson
Active ingredient: Guselkumab
Applicant: Johnson & Johnson
Indication: Psoriasis
Mechanism of action IL-23 inhibitor
Wholesale acquisition cost*: $58,100/year
Incentive: Priority review voucher filed with application
Nerlynx (Small Molecule)
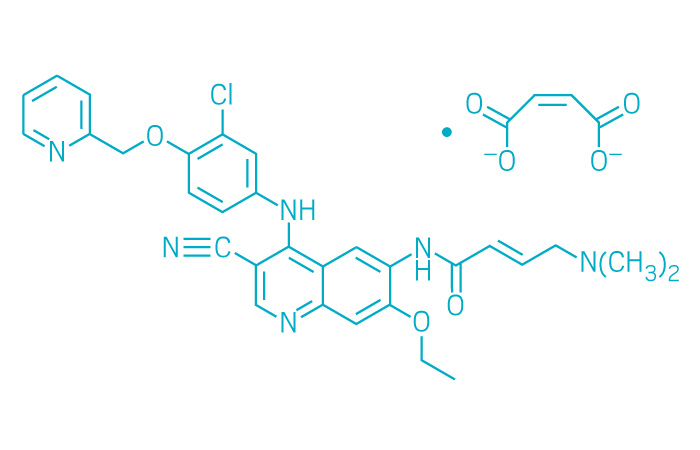
Active ingredient: Neratinib maleate
Applicant: Puma Biotechnology
Indication: HER2-positive breast cancer
Mechanism of action EGFR, HER2, and HER4 inhibitor
Wholesale acquisition cost*: $10,500/month
Vosevi (Small Molecule)
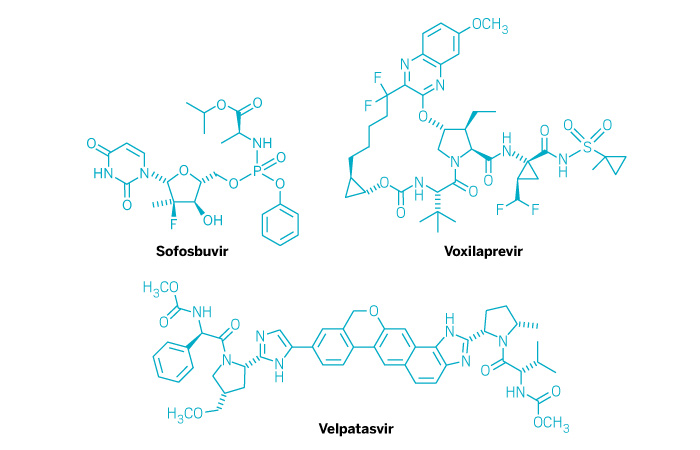
Active ingredient: Sofosbuvir, velpatasvir, and voxilaprevir
Applicant: Gilead Sciences
Indication: Hepatitis C
Mechanism of action NS5B polymerase, NS5B, and NS3/4A protease inhibitors
Wholesale acquisition cost*: $74,760/12-week course
FDA special status: Breakthrough
Idhifa (Small Molecule)
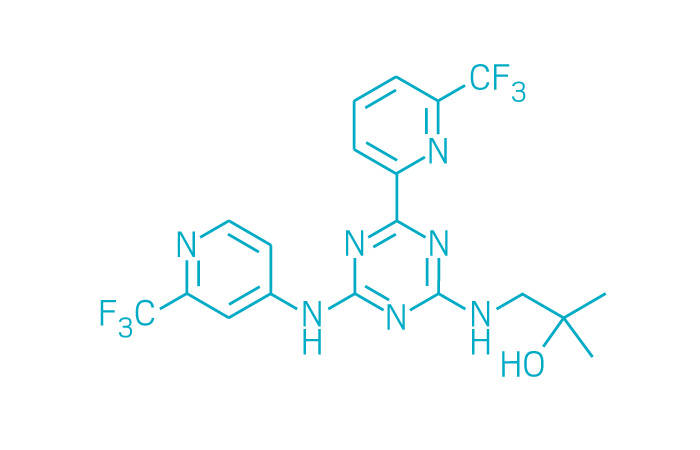
Active ingredient: Enasidenib
Applicant: Celgene
Indication: IDH2-positive acute myeloid leukemia
Mechanism of action IDH2 inhibitor
Wholesale acquisition cost*: $24,872/month
Mavyret (Small Molecule)
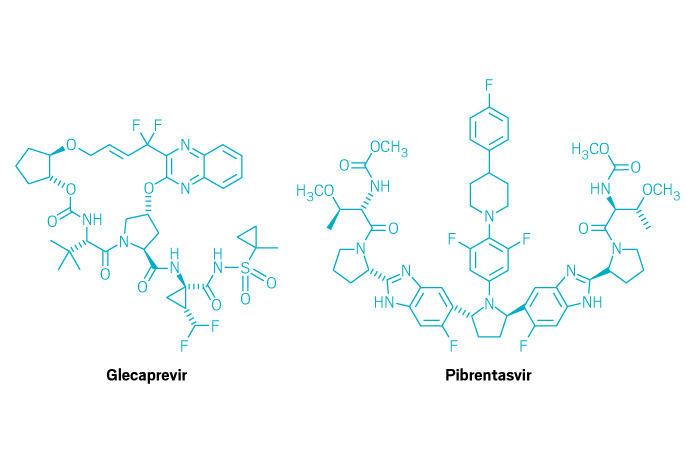
Active ingredient: Glecaprevir and pibrentasvir
Applicant: AbbVie
Indication: Hepatitis C
Mechanism of action NS3/4A protease and NS5A inhibitors
Wholesale acquisition cost*: $26,400/8-week course
FDA special status: Breakthrough
Besponsa (Antibody-Drug Conjugate)

Credit: Pfizer
Active ingredient: Inotuzumab ozogamicin
Applicant: Pfizer
Indication: Acute lymphoblastic leukemia
Mechanism of action CD22-binding antibody and cytotoxic antibiotic
Wholesale acquisition cost*: N/A
FDA special status: Breakthrough
Vabomere (Small Molecule)
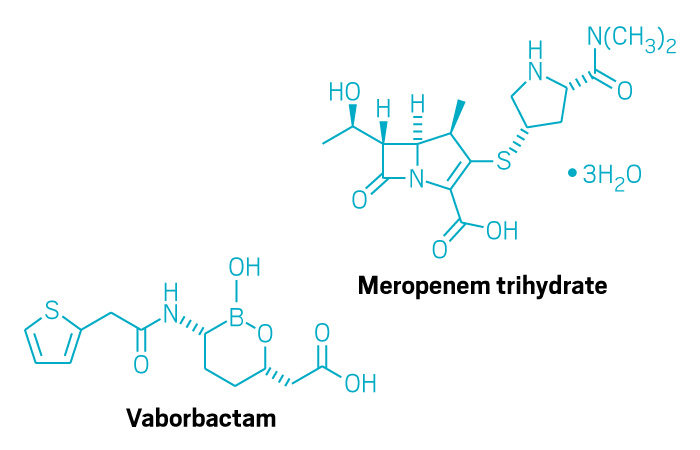
Active ingredient: Meropenem and vaborbactam
Applicant: The Medicines Co.
Indication: Complicated urinary tract infections
Mechanism of action Carbapenem antibacterial and non-β-lactam β-lactamase inhibitor
Wholesale acquisition cost*: N/A
Benznidazole (Small Molecule)
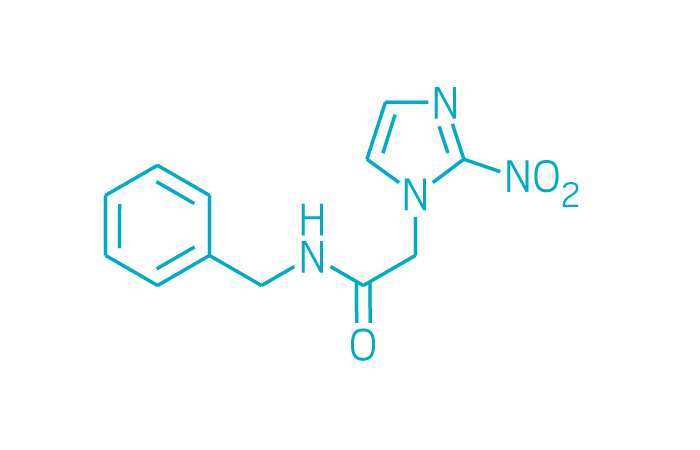
Active ingredient: Benznidazole
Applicant: Chemo Research
Indication: Chagas disease
Mechanism of action Nitroimidazole antimicrobial
Wholesale acquisition cost*: N/A
FDA special status: Accelerated approval
Incentive: Tropical disease priority review voucher
Aliqopa (Small Molecule)
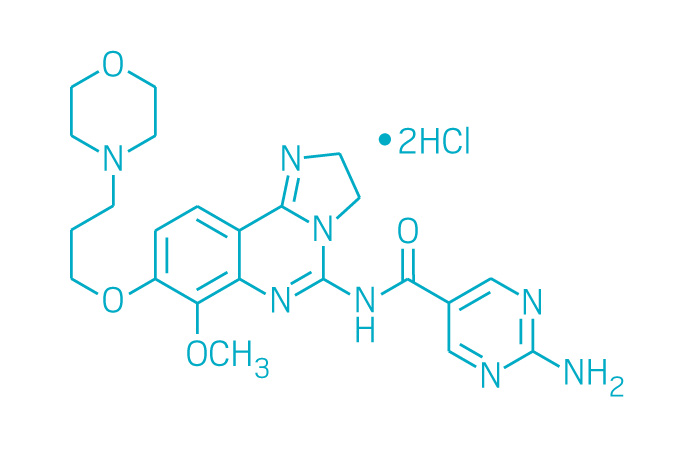
Active ingredient: Copanlisib
Applicant: Bayer
Indication: Follicular lymphoma
Mechanism of action Pan-PI3K inhibitor
Wholesale acquisition cost*: $12,726/month
FDA special status: Accelerated approval
Solosec (Small Molecule)
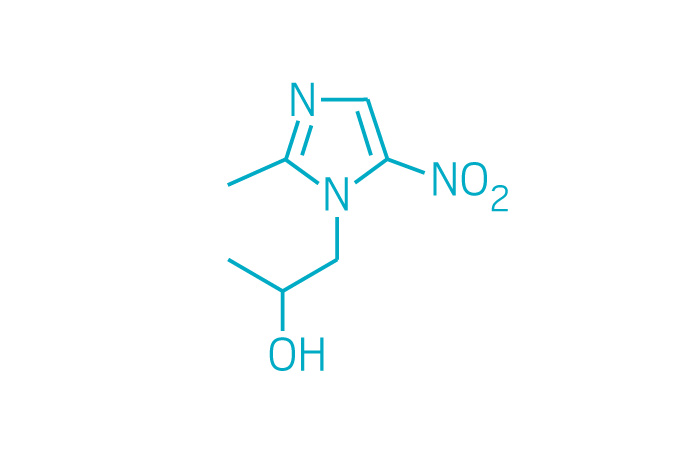
Active ingredient: Secnidazole
Applicant: Symbiomix Therapeutics
Indication: Bacterial vaginosis
Mechanism of action Nitroimidazole antimicrobial
Wholesale acquisition cost*: N/A
Verzenio (Small Molecule)
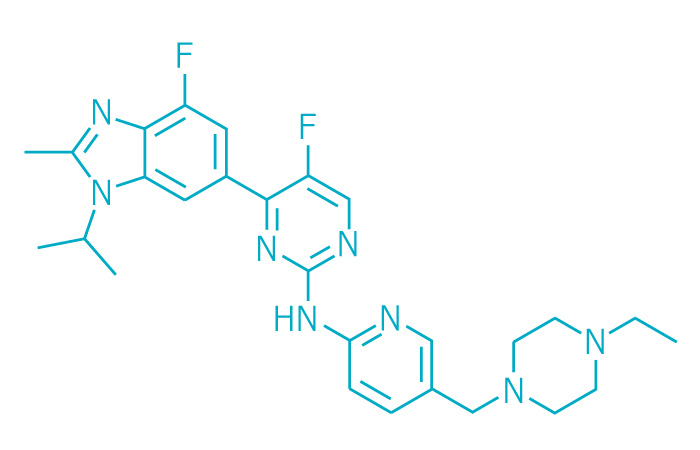
Active ingredient: Abemaciclib
Applicant: Eli Lilly & Co.
Indication: Breast cancer
Mechanism of action CDK4/6 inhibitor
Wholesale acquisition cost*: $10,948/month
FDA special status: Breakthrough
Calquence (Small Molecule)
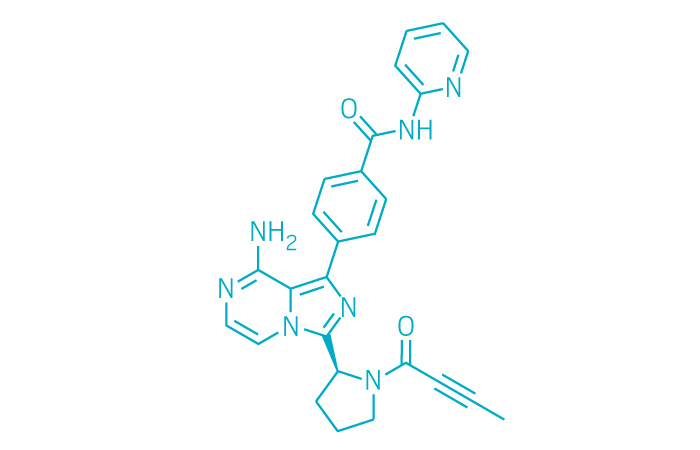
Active ingredient: Acalabrutinib
Applicant: AstraZeneca
Indication: Mantle cell lymphoma
Mechanism of action BTK inhibitor
Wholesale acquisition cost*: $14,259/month
FDA special status: Breakthrough | Accelerated approval
Vyzulta (Small Molecule)
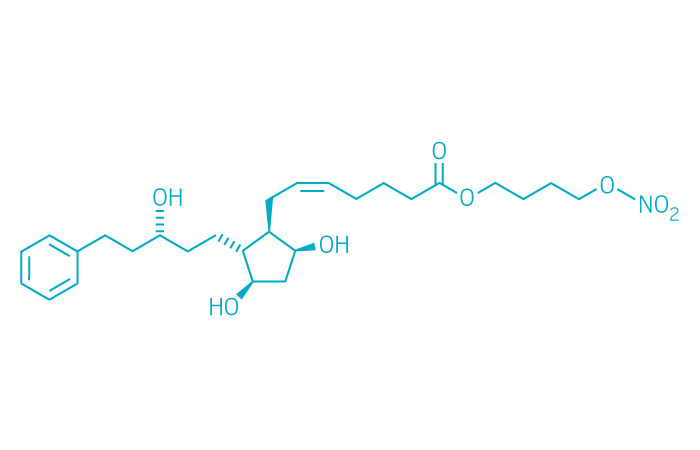
Active ingredient: Latanoprostene bunod ophthalmic solution
Applicant: Valeant Pharmaceuticals
Indication: Glaucoma/ocular hypertension
Mechanism of action Metabolizes into two pressure-lowering moieties
Wholesale acquisition cost*: N/A
Prevymis (Small Molecule)
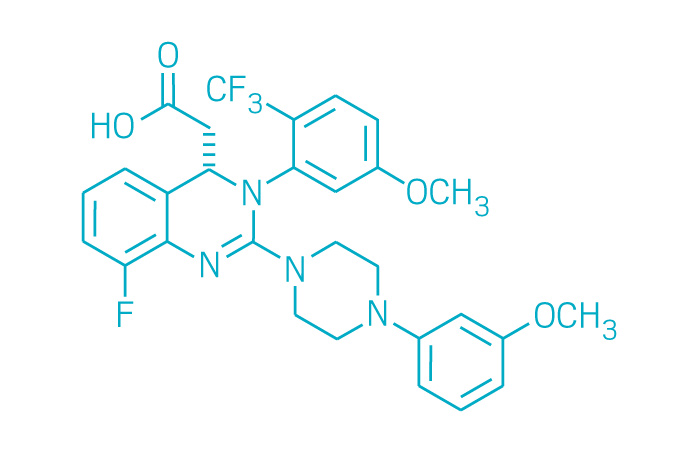
Active ingredient: Letermovir
Applicant: Merck & Co.
Indication: Infection prevention after bone marrow transplant
Mechanism of action Cytomegalovirus DNA terminase complex inhibitor
Wholesale acquisition cost*: $195/day for tablets or $270/injection
FDA special status: Breakthrough
Fasenra (Antibody)

Credit: AstraZeneca
Active ingredient: Benralizumab
Applicant: AstraZeneca
Indication: Severe asthma
Mechanism of action IL-5R α receptor binder
Wholesale acquisition cost*: $38,000/first year
Mepsevii (Enzyme)

Credit: Ultragenyx Pharmaceutical
Active ingredient: Vestronidase alfa
Applicant: Ultragenyx Pharmaceutical
Indication: MPS VII (Sly syndrome)
Mechanism of action Enzyme replacement therapy
Wholesale acquisition cost*: $375,000/year
Incentive: Rare disease priority review voucher, sold to Novartis for $130 million
Hemlibra (Bispecific Antibody)
Credit: Roche
Active ingredient: Emicizumab
Applicant: Roche
Indication: Hemophilia A
Mechanism of action Factor IX and X binder
Wholesale acquisition cost*: $482,000/first year
FDA special status: Breakthrough
Ozempic (Peptide)
Credit: Novo Nordisk
Active ingredient: Semaglutide
Applicant: Novo Nordisk
Indication: Type 2 diabetes
Mechanism of action GLP-1 receptor agonist
Wholesale acquisition cost*: $676/4–6 week supply
Xepi (Small Molecule)
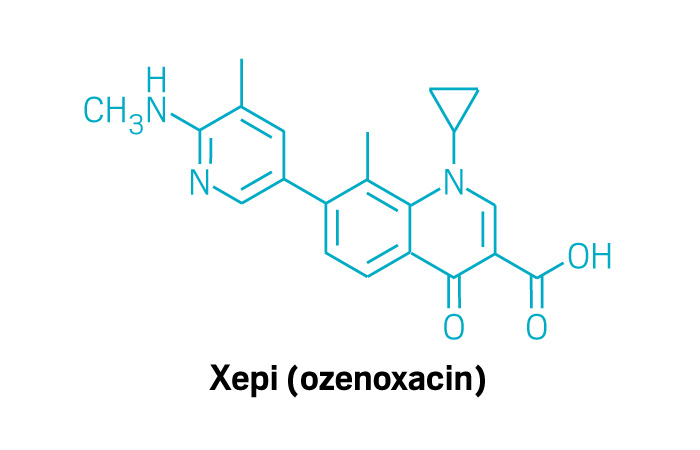
Active ingredient: Ozenoxacin
Applicant: Medimetriks Pharmaceuticals
Indication: Impetigo
Mechanism of action Nonfluorinated quinolone antibiotic
Wholesale acquisition cost*: N/A
Rhopressa (Small Molecule)
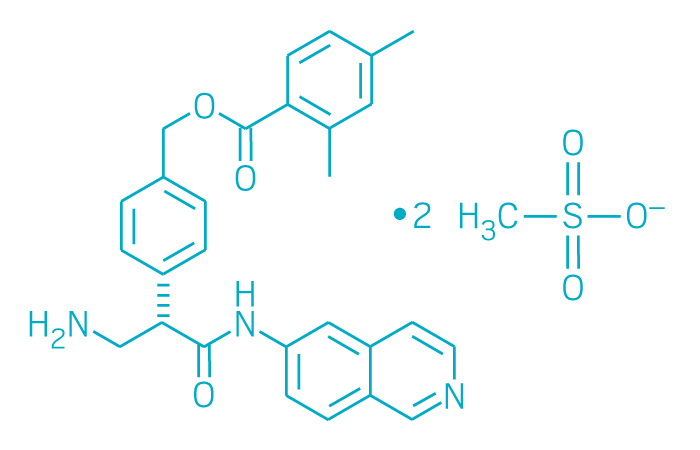
Active ingredient: Netarsudil ophthalmic solution
Applicant: Aerie Pharmaceuticals
Indication: Glaucoma/ocular hypertension
Mechanism of action ρ-Kinase inhibitor
Wholesale acquisition cost*: N/A
Macrilen (Small Molecule)
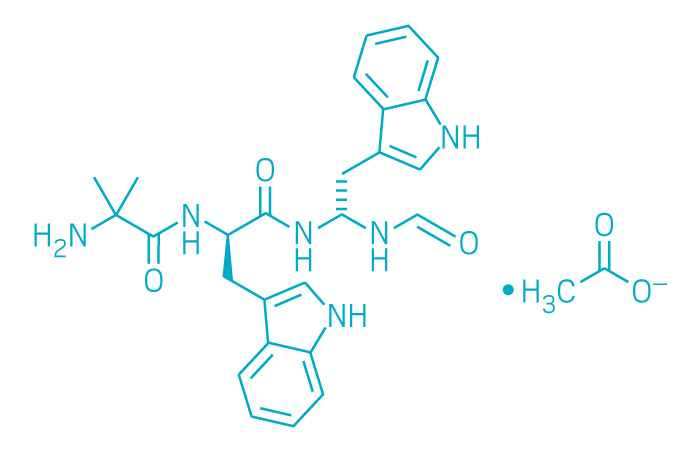
Active ingredient: Macimorelin acetate
Applicant: Aeterna Zentaris
Indication: Adult growth hormone deficiency
Mechanism of action Ghrelin mimetic
Wholesale acquisition cost*: N/A
Steglatro (Small Molecule)
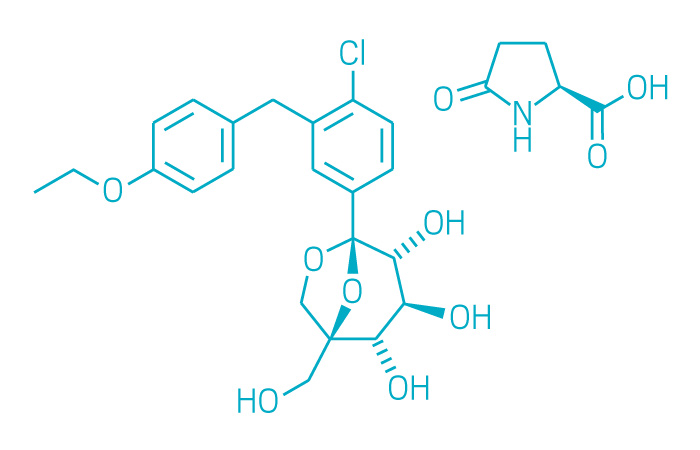
Active ingredient: Ertugliflozin
Applicant: Merck & Co./Pfizer
Indication: Type 2 diabetes
Mechanism of action SGLT2 inhibitor
Wholesale acquisition cost*: N/A
Giapreza (Peptide)

Credit: La Jolla Pharmaceutical
Active ingredient: Angiotensin II
Applicant: La Jolla Pharmaceutical
Indication: Hypotension in sepsis or critical illness
Mechanism of action Synthetic human angiotensin II
Wholesale acquisition cost*: N/A
Pfizer, which in 2016 had a lone approval that came only through a midyear acquisition, also scored three new products last year. However, it must share rights to two of them: the cancer immunotherapy Bavencio, which came to Pfizer in 2014 through a multi-billion-dollar deal with Germany’s Merck KGaA, and the diabetes treatment Steglatro, an internally developed drug that in 2013 Pfizer chose to codevelop with U.S.-based Merck & Co.
For others, the drought persisted. Although Bristol-Myers Squibb continued to expand the types of cancer that are treated with its existing immuno-oncology drugs, 2017 was the company’s second year in a row without a new product approval.
The drug industry’s record-breaking productivity does come with caveats. Although FDA said 33% of the products it approved belonged to new classes of compounds, most drug industry watchers would consider several of them to be older drugs.
For example, FDA’s list of first-in-class drugs includes Emflaza, a decades-old corticosteroid approved for the first time in the U.S. to treat Duchenne muscular dystrophy. A handful of other treatments either are similarly old drugs just now reaching the U.S. market or work against older protein targets now associated with new diseases.
Cancer treatments continued to dominate the list, representing over a quarter of all new molecules approved last year. However, many of the new oncology drugs are not especially unique and work by the same mechanism of action as already marketed drugs. The approval list included two more CDK4/6 inhibitors, two more PD-L1 inhibitors, and more compounds that block the proteins PARP, BTK, and ALK.
The new cancer treatments were the primary beneficiaries of FDA’s effort to speed development of potentially important new drugs. Of the 12 oncology products approved, nine had breakthrough therapy designation (BTD), a status introduced in 2012. FDA has previously described BTD as triggering an all-hands-on-deck approach at the agency to ensure efficient and well-designed clinical programs for drugs that are novel or address an underserved disease.
The program has been growing quickly. Overall, 17 drugs approved in 2017 had BTD status. In 2015, which previously held the recent record for new drug approvals, just 10 products had the special status.
Beyond cancer, anti-infectives and drugs for rare diseases were the other beneficiaries of breakthrough status. According to the Tufts Center for the Study of Drug Development, BTD is significantly shortening drug development timelines. The average time from Investigational New Drug Application—asking FDA to begin clinical trials—to approval letter was 65 months for the 17 BTD drugs approved in 2015 and 2016, compared with 110 months for drugs approved without the status.
“I would say that’s pretty good confirmation that this is another FDA program that seems to be working,” says Christopher-Paul Milne, director of research at the Tufts center. “What has to come next is expansion into other therapeutic areas of need.”
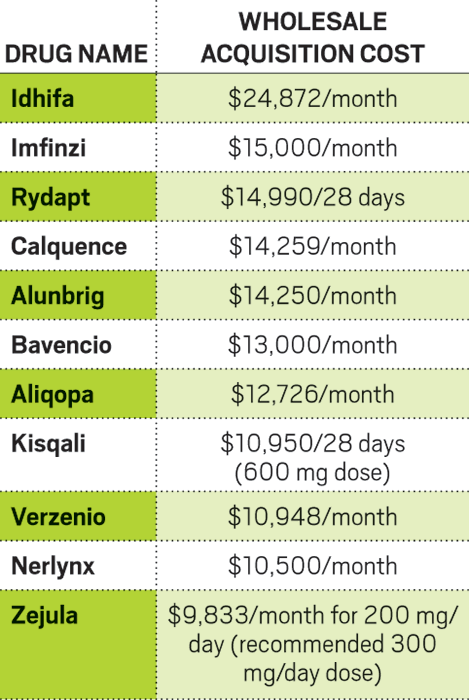
That shortened development time, which in theory should mean lower R&D costs for companies focused on oncology, is not translating into lower prices. All 12 oncology drugs approved last year featured six-figure annual wholesale acquisition costs, the company’s list price before any rebates or discounts.
Although the biotech industry has been energized by the potential for new technologies and therapeutic modalities to tackle difficult-to-treat diseases, small molecules continue to dominate FDA’s docket. Conventional, chemically synthesized drugs represented almost two-thirds of the new molecular entities approved last year.
Still, compared with a decade ago, the new drug list last year featured a wider range of modalities, including peptides, enzyme replacement therapies for rare diseases, and an antibody-drug conjugate.
Industry insiders will notice that the tally of 46 does not include the two new therapeutic modalities that garnered the biggest headlines in 2017. Missing are Novartis’s in pharma Kymriah and Gilead Sciences’ Yescarta, both chimeric antigen receptor (CAR) T-cell therapies, a new class of drugs made by reengineering a person’s own T cells to seek and destroy cancer cells. Also absent is Spark Therapeutics’ Luxturna, the first-ever approved gene therapy for a genetic condition. Luxturna treats a rare form of blindness.
C&EN has long tracked FDA’s actions on new molecular entities, and these cellular treatments—the approval of which the agency called “historic”—fall outside that category. Overall, FDA gave the green light to a combined 56 new molecular entities and biologic therapies.
Similarly, the list does not capture another groundbreaking approval that arrived in 2017. In May, FDA green-lighted Merck & Co.’s cancer immunotherapy Keytruda for use in anyone harboring a specific genetic profile.
Keytruda had already been on the market for three years for a variety of cancer types. But last year the agency gave the drug its first “tissue agnostic” approval, meaning that a genetic mutation rather than the location of the cancer—lung or colon, for example—guides use of the treatment.
Soon after coming on as FDA commissioner on May 9, Scott Gottlieb signaled his intention to ease the path for other tissue-agnostic drugs, and he quickly acted: In December, the agency released draft guidance to clarify the clinical development for such treatments.
“When drugs successfully target these molecular mistakes to reverse the effects of different diseases, we need a development pathway that allows the new drug to pursue approval in each of these novel settings on the basis of the molecular marker that the drug targets,” Gottlieb said when the draft guidance was released.
Even as the breadth of modalities and clinical pathways expands, the agency said that every drug in 2017 was approved within the review time frame required by law; many applications actually got the nod before the deadline.
Looking ahead, the agency appears determined to continue partnering with companies to make the development process for innovative drugs even more efficient.