Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Theoretical Chemistry
Amazing Women
Brenda Rubenstein
Quantum connoisseur is improving molecular simulations’ speed and accuracy
by Sam Lemonick
August 25, 2019
| A version of this story appeared in
Volume 97, Issue 33


Vitals
Current affiliation: Brown University
Age: 33
PhD alma mater: Columbia University
If I were an element, I would be: “Oxygen. It brings out the best and most exotic from other elements while making life—and therefore science—possible.”
If I weren’t a chemist, I would be: “Mountaineer. If I could hike and climb in idyllic places that grant me the peace of mind to think deeply for reasonable pay, believe me, I would do so in a heartbeat.”
Must-have in the lab: “Wonder. We pursue a number of challenging and unusual research directions in my group; it is wonder that keeps us motivated and hungry despite the steep climbs.”
Must-have on the road: “Look at my hair—clearly, I can never have enough brushes!”
3 key papers
“Encoding Information in Synthetic Metabolomes” (bioRxiv 2019, DOI: 10.1101/627745)
“Ab Initio Finite Temperature Auxiliary Field Quantum Monte Carlo” (J. Chem. Theory Comput. 2018, DOI: 10.1021/acs.jctc.8b00569)
“The Role of Extracellular Matrix in Glioma Invasion: A Cellular Potts Model Approach” (Biophys. J. 2008, DOI: 10.1529/biophysj.108.140624)
Here’s the problem with using computers to model chemistry: The methods that can give you the most detailed and accurate predictions are slow and take a lot of computer power. The tools that give answers more quickly don’t always get them quite right. So what’s a chemist to do?
Brown University’s Brenda Rubenstein is working on one solution. She’s refining quantum Monte Carlo simulation techniques so chemists can make better predictions about systems in which electrons matter, like superconductors, batteries, and catalysts.
Monte Carlo methods can make accurate predictions about a system according to random sampling. As a simple example, think of predicting 100 flips of a two-sided coin. Rather than flipping it 100 times, a smaller number of flips can simulate the whole set. These methods are especially useful in situations with lots of interrelated variables, which can frustrate other modeling approaches. The quantum mechanics of atoms and electrons are that kind of complex system, and Monte Carlo techniques aim to use models to predict these mechanics.
Rubenstein has helped adapt quantum Monte Carlo methods that are common in physics to chemistry. One focus is finding ways around something called the sign problem, in which some probabilities are negative—an issue that has plagued chemical applications of quantum Monte Carlo methods. And she’s applied the technique to systems at temperatures above absolute zero. Most quantum Monte Carlo simulations work best at 0 K right now.
“She’s very creative, very fearless,” says Rubenstein’s PhD mentor, Columbia University’s David Reichman. “To do difficult research like she does, you have to have those qualities.”
Rubenstein is also working to extend quantum Monte Carlo to larger systems. Right now, modeling systems with hundreds of electrons is pushing the technique’s limits, and she says, “I’m talking about doing millions of electrons, millions of orbitals.” If Rubenstein can help make Monte Carlo methods work better for chemistry, they could become a routine tool in chemists’ modeling inventory.
But Rubenstein’s work doesn’t stop at quantum Monte Carlo. She recently ventured into making chemical computers. Rubenstein explains that transistors—the basic building blocks of computer chips—are at a point where they can’t get much smaller at a time when we’re creating more information than we have space to store. Chemicals can both record information and do computational operations without taking up much space.
With her colleagues at Brown, she’s shown how mixtures of chemicals can perform basic computational tasks used in some artificial intelligence algorithms. The group has also worked out a way to encode information in mixtures of synthetic metabolites so they act similarly to the ones and zeros in data storage.
On top of all that, Rubenstein chairs her department’s Diversity and Inclusion Action Committee, part of a university-wide effort started in 2016. And for the past few years she has mentored underrepresented and low-income students through high school science fairs as part of a program run by the Society for Science & the Public.
If that all sounds like a lot, it is. And Rubenstein feels the weight of her responsibility. When you become a professor, she says, “you have to perform and behave better than you otherwise would.”
Here’s the problem with using computers to model chemistry: The methods that can give you the most detailed and accurate predictions are slow and take a lot of computer power. The tools that give answers more quickly don’t always get them quite right. So what’s a chemist to do?
Brown University’s Brenda Rubenstein is working on one solution. She’s refining quantum Monte Carlo simulation techniques so chemists can make better predictions about systems in which electrons matter, like superconductors, batteries, and catalysts.
Advertisement
Monte Carlo methods can make accurate predictions about a system according to random sampling. As a simple example, think of predicting 100 flips of a two-sided coin. Rather than flipping it 100 times, a smaller number of flips can simulate the whole set. These methods are especially useful in situations with lots of interrelated variables, which can frustrate other modeling approaches. The quantum mechanics of atoms and electrons are that kind of complex system, and Monte Carlo techniques aim to use models to predict these mechanics.
Rubenstein has helped adapt quantum Monte Carlo methods that are common in physics to chemistry. One focus is finding ways around something called the sign problem, in which some probabilities are negative—an issue that has plagued chemical applications of quantum Monte Carlo methods. And she’s applied the technique to systems at temperatures above absolute zero. Most quantum Monte Carlo simulations work best at 0 K right now.
“She’s very creative, very fearless,” says Rubenstein’s PhD mentor, Columbia University’s David Reichman. “To do difficult research like she does, you have to have those qualities.”
Rubenstein is also working to extend quantum Monte Carlo to larger systems. Right now, modeling systems with hundreds of electrons is pushing the technique’s limits, and she says, “I’m talking about doing millions of electrons, millions of orbitals.” If Rubenstein can help make Monte Carlo methods work better for chemistry, they could become a routine tool in chemists’ modeling inventory.
But Rubenstein’s work doesn’t stop at quantum Monte Carlo. She recently ventured into making chemical computers. Rubenstein explains that transistors—the basic building blocks of computer chips—are at a point where they can’t get much smaller at a time when we’re creating more information than we have space to store. Chemicals can both record information and do computational operations without taking up much space.
With her colleagues at Brown, she’s shown how mixtures of chemicals can perform basic computational tasks used in some artificial intelligence algorithms. The group has also worked out a way to encode information in mixtures of synthetic metabolites so they act similarly to the ones and zeros in data storage.
On top of all that, Rubenstein chairs her department’s Diversity and Inclusion Action Committee, part of a university-wide effort started in 2016. And for the past few years she has mentored underrepresented and low-income students through high school science fairs as part of a program run by the Society for Science & the Public.
If that all sounds like a lot, it is. And Rubenstein feels the weight of her responsibility. When you become a professor, she says, “you have to perform and behave better than you otherwise would.”
Vitals
Current affiliation: Brown University
Age: 33
PhD alma mater: Columbia University
If I were an element, I would be: “Oxygen. It brings out the best and most exotic from other elements while making life—and therefore science—possible.”
If I weren’t a chemist, I would be: “Mountaineer. If I could hike and climb in idyllic places that grant me the peace of mind to think deeply for reasonable pay, believe me, I would do so in a heartbeat.”
Must-have in the lab: “Wonder. We pursue a number of challenging and unusual research directions in my group; it is wonder that keeps us motivated and hungry despite the steep climbs.”
Must-have on the road: “Look at my hair—clearly, I can never have enough brushes!”
Research at a glance
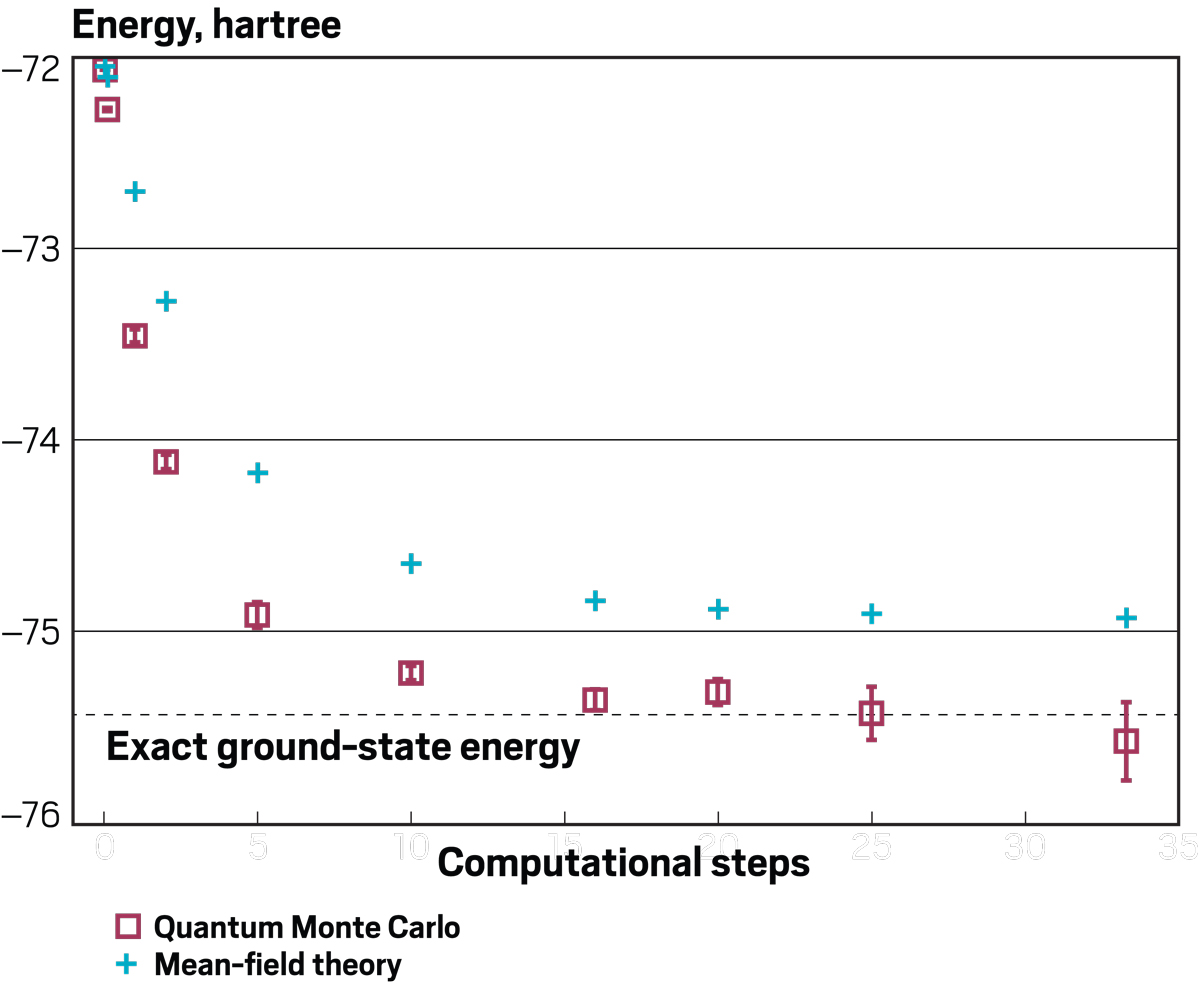
Credit: Adapted from J. Chem. Theory Comput./Yang H. Ku/C&EN
Rubenstein uses quantum Monte Carlo methods to predict the exact energy of a system, in this case C2 molecules, faster and more accurately than other methods.
Three key papers
“Encoding Information in Synthetic Metabolomes”
(bioRxiv 2019, DOI: 10.1101/627745)
“Ab Initio Finite Temperature Auxiliary Field Quantum Monte Carlo”
(J. Chem. Theory Comput. 2018, DOI: 10.1021/acs.jctc.8b00569)

















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter