Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.

Biotechnology
The emergence of biologics
As the fields of genetics and molecular biology evolved over the 20th century, scientists set out to use cell and genetic engineering to create medicines. These 5 events helped lay the foundation for a new therapeutics industry
by Alla Katsnelson, special to C&EN
August 11, 2023
| A version of this story appeared in
Volume 101, Issue 26
1976: Genentech launches

Biochemist Herbert W. Boyer (left) and venture capitalist Robert A. Swanson (right) founded Genentech in 1976.
Credit: Genentech
Genentech’s founding is a legend in the biotechnology world: Two scientists meet at a conference in Hawaii in 1972. Over hot pastrami and corned beef sandwiches, they hatch the idea of using a DNA-cleaving enzyme to insert specific genes into bacterial plasmids. Their plan—now known as recombinant DNA technology—works, and 4 years later a venture capitalist persuades one of them to commercialize it.
The fledgling firm, widely considered the first biotech company, and the biotechs that followed quickly gained reputations as places to do paradigm-shifting research with few intellectual or financial restrictions. “There was nobody above us—no VP of research,” recalls Richard Lawn, who joined Genentech as employee number 44 in 1980. In meetings, scientists sketched out their best guesses for which chunks of bacterial DNA drove gene expression or acted as stop signals. “This stuff sounds so trivial in retrospect, but nobody had done it before,” Lawn says.
That first wave of activity laid the foundation for the industry’s evolution. Today, there are thousands of biotech companies around the world.
1982: The FDA approves recombinant insulin
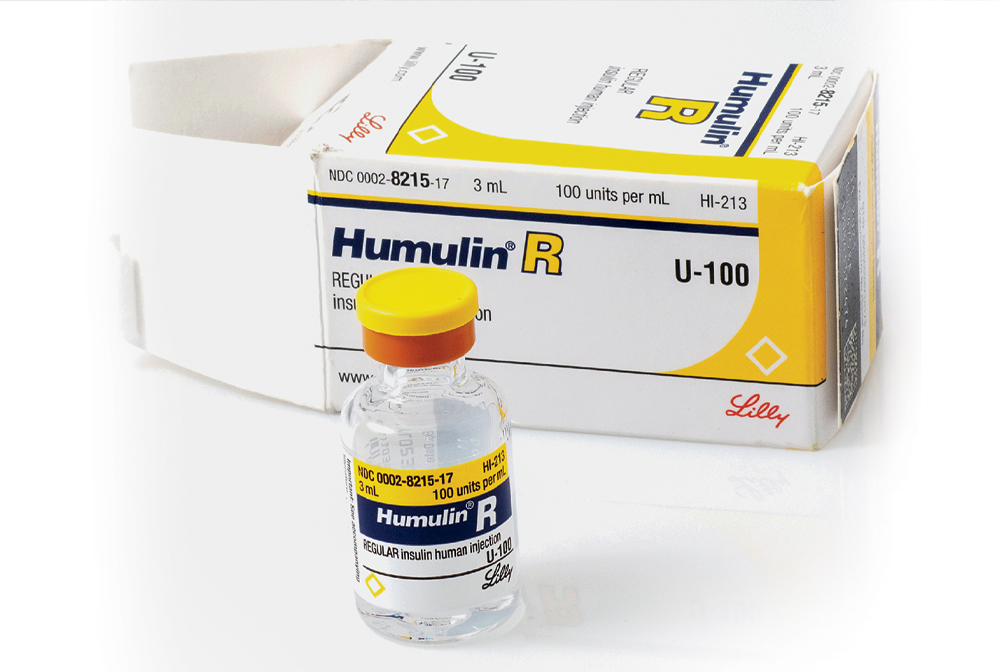
Genentech succeeded in producing insulin using its recombinant technology in 1978. Eli Lilly and Company licensed the drug the same year.
Credit: Badon Hill Studio/Shutterstock
Discovered in 1921, insulin quickly transformed the health of millions. But its supply was limited because it was extracted from the glands of pigs and other animals. Scientists at Genentech set out to produce it using recombinant DNA technology, and in 1978 they succeeded, by inserting the gene encoding it into the bacterium Escherichia coli. The US Food and Drug Administration approved the drug—called Humulin and licensed by Eli Lilly and Company—4 years later.
Chemical expertise played an underappreciated role in making Humulin commercially viable; it scaled up and industrialized the drug’s production from milligram to kilogram quantities, says Richard DiMarchi, a chemist at Indiana University Bloomington who worked on Humulin’s development when he started as a research scientist at Lilly in 1981. The drug’s success validated the notion that recombinant therapies could be profitable.
In 1985, the FDA approved a second Genentech-made recombinant protein, a growth hormone called Protropin. It later approved Amgen’s recombinant erythropoietin, called Epogen. A hormone that stimulates the growth of red blood cells, Epogen became biotechnology’s first blockbuster drug.
1986: The FDA approves the first monoclonal antibody therapy
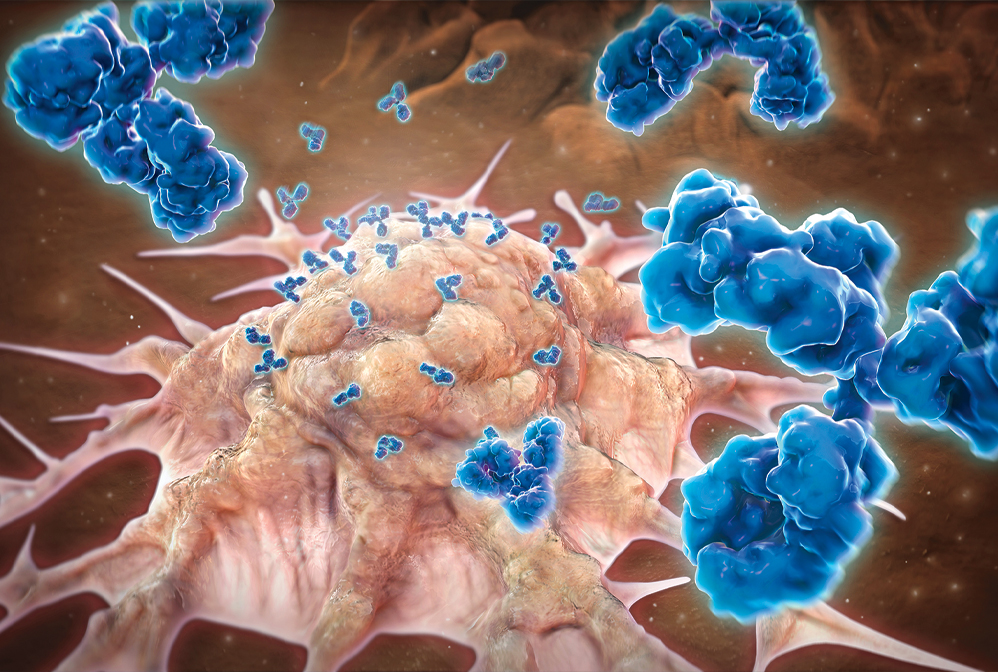
Monoclonal antibodies, which make up a large slice of the biologics market, target proteins on specific cell types to mark them for destruction by the immune system. Shown here is a cancer cell.
Credit: Evan Oto/Science Source
Monoclonal antibody therapies, which stimulate the immune system by binding to specific cells or proteins, had a rocky path to success. Georges J. F. Köhler and César Milstein first produced antibodies with known specificity in 1975, creating so-called hybridoma cell lines that could pump out antibodies indefinitely (DOI: doi.org/10.1038/256495a0). Within a few years, researchers grasped the technique’s potential for making medicines. In 1986, the US Food and Drug Administration approved the first monoclonal antibody drug, an antibody called OKT3 that prevented the rejection of transplanted organs. But because OKT3 was made in mice, not humans, it often triggered an immune response in patients.
Eight years passed before the FDA approved the next monoclonal antibody drug, and successes slowly accrued as researchers found ways to make human versions of these molecules. Today, more than 160 monoclonal antibody therapies have been approved worldwide for a wide range of indications; over 40% target cancer (DOI: doi.org/10.1038/256495a0).
1990: The Human Genome Project starts

Francis Collins, who served as director of the Human Genome Project, announces the project’s completion in 2003.
Credit: Associated Press
The $2.7 billion international effort to sequence the human genome ran from 1990 to 2003 and concluded at about 90% completion. The idea of devoting such enormous resources to sequencing a single genome looks almost quaint in hindsight, but that’s largely because the project spurred the development of a huge number of technologies that molecular biologists and biological chemists now use daily for drug discovery. The effort also fueled the creation of an infrastructure for multi-institutional, big-data research and of course pushed society to reimagine ideas of disease and health in genomic and bioinspired terms.
1999: Jesse Gelsinger dies in gene therapy trial
Jesse Gelsinger was a participant in a clinical trial. He died in 1999 after receiving a gene therapy packaged in an adenovirus.
Credit: Shutterstock
Ask any biotechnology professional about the industry’s seismic moments, and most will name the death of 18-year-old Jesse Gelsinger, a participant in a gene therapy clinical trial run by James Wilson at the University of Pennsylvania. The event sent shock waves through the biomedical research community. The US Food and Drug Administration suspended the trial and scrutinized dozens of other gene therapy trials, and the incident led regulators to revamp the ethical framework surrounding clinical trial participation.
But researchers regrouped, and within a decade they had developed and tested safer vectors made of adeno-associated viruses and lentiviruses. Those efforts are now bearing fruit. In 2017, a treatment for a genetic form of blindness became the first FDA-approved gene therapy for a genetic disease, and more than a dozen gene therapy approvals have followed. The gene-editing technology CRISPR has brought our ability to alter genes to a whole new level, making it possible not just to add working versions of faulty genes but to fix mistakes in the genome.
Alla Katsnelson is a freelance science writer and editor in Southampton, Massachusetts.





1976: Genentech launches

Genentech’s founding is a legend in the biotechnology world: Two scientists meet at a conference in Hawaii in 1972. Over hot pastrami and corned beef sandwiches, they hatch the idea of using a DNA-cleaving enzyme to insert specific genes into bacterial plasmids. Their plan—now known as recombinant DNA technology—works, and 4 years later a venture capitalist persuades one of them to commercialize it.
The fledgling firm, widely considered the first biotech company, and the biotechs that followed quickly gained reputations as places to do paradigm-shifting research with few intellectual or financial restrictions. “There was nobody above us—no VP of research,” recalls Richard Lawn, who joined Genentech as employee number 44 in 1980. In meetings, scientists sketched out their best guesses for which chunks of bacterial DNA drove gene expression or acted as stop signals. “This stuff sounds so trivial in retrospect, but nobody had done it before,” Lawn says.
That first wave of activity laid the foundation for the industry’s evolution. Today, there are thousands of biotech companies around the world.
1982: The FDA approves recombinant insulin

Discovered in 1921, insulin quickly transformed the health of millions. But its supply was limited because it was extracted from the glands of pigs and other animals. Scientists at Genentech set out to produce it using recombinant DNA technology, and in 1978 they succeeded, by inserting the gene encoding it into the bacterium Escherichia coli. The US Food and Drug Administration approved the drug—called Humulin and licensed by Eli Lilly and Company—4 years later.
Chemical expertise played an underappreciated role in making Humulin commercially viable; it scaled up and industrialized the drug’s production from milligram to kilogram quantities, says Richard DiMarchi, a chemist at Indiana University Bloomington who worked on Humulin’s development when he started as a research scientist at Lilly in 1981. The drug’s success validated the notion that recombinant therapies could be profitable.
In 1985, the FDA approved a second Genentech-made recombinant protein, a growth hormone called Protropin. It later approved Amgen’s recombinant erythropoietin, called Epogen. A hormone that stimulates the growth of red blood cells, Epogen became biotechnology’s first blockbuster drug.
1986: The FDA approves the first monoclonal antibody therapy

Monoclonal antibody therapies, which stimulate the immune system by binding to specific cells or proteins, had a rocky path to success. Georges J. F. Köhler and César Milstein first produced antibodies with known specificity in 1975, creating so-called hybridoma cell lines that could pump out antibodies indefinitely. Within a few years, researchers grasped the technique’s potential for making medicines. In 1986, the US Food and Drug Administration approved the first monoclonal antibody drug, an antibody called OKT3 that prevented the rejection of transplanted organs. But because OKT3 was made in mice, not humans, it often triggered an immune response in patients.
Eight years passed before the FDA approved the next monoclonal antibody drug, and successes slowly accrued as researchers found ways to make human versions of these molecules. Today, more than 160 monoclonal antibody therapies have been approved worldwide for a wide range of indications; over 40% target cancer.
1990: The Human Genome Project starts

The $2.7 billion international effort to sequence the human genome ran from 1990 to 2003 and concluded at about 90% completion. The idea of devoting such enormous resources to sequencing a single genome looks almost quaint in hindsight, but that’s largely because the project spurred the development of a huge number of technologies that molecular biologists and biological chemists now use daily for drug discovery. The effort also fueled the creation of an infrastructure for multi-institutional, big-data research and of course pushed society to reimagine ideas of disease and health in genomic and bioinspired terms.
1999: Jesse Gelsinger dies in gene therapy trial
Ask any biotechnology professional about the industry’s seismic moments, and most will name the death of 18-year-old Jesse Gelsinger, a participant in a gene therapy clinical trial run by James Wilson at the University of Pennsylvania. The event sent shock waves through the biomedical research community. The US Food and Drug Administration suspended the trial and scrutinized dozens of other gene therapy trials, and the incident led regulators to revamp the ethical framework surrounding clinical trial participation.
But researchers regrouped, and within a decade they had developed and tested safer vectors made of adeno-associated viruses and lentiviruses. Those efforts are now bearing fruit. In 2017, a treatment for a genetic form of blindness became the first FDA-approved gene therapy for a genetic disease, and more than a dozen gene therapy approvals have followed. The gene-editing technology CRISPR has brought our ability to alter genes to a whole new level, making it possible not just to add working versions of faulty genes but to fix mistakes in the genome.
Alla Katsnelson is a freelance science writer and editor in Southampton, Massachusetts.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter