Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Infectious disease
Covid-19
How big pharma firms are quietly collaborating on new coronavirus antivirals
Behind the scenes, companies including Novartis, Takeda, and Gilead are collaborating in a loose alliance. Their work might not be done in time to stop COVID-19, but they hope it can prevent the next pandemic
by Lisa M. Jarvis
May 1, 2020
| A version of this story appeared in
Volume 98, Issue 18

The early weeks of the coronavirus pandemic were a scramble as infectious disease experts looked for anything in the medicine cabinet that might work to treat COVID-19. Clinicians tried drugs designed for other viruses—HIV, Ebola virus, influenza viruses—hoping that the proteins they target would be similar enough to those in this virus, SARS-CoV-2.
Those efforts get you only so far. Viruses have subtle differences in their machinery, not to mention the way they wreak havoc on the body. Even when a drug offers modest benefits, it might not be suitable for widespread use. For example, Gilead Sciences’ antiviral remdesivir, originally developed for Ebola, appears to help some people with COVID-19 recover faster. But it is also an intravenous drug and so can’t be used to prevent people from getting sick.
The medicine chest needs more options, ideally ones tailor made for this coronavirus.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe
In the past 6 weeks, big pharma firms have begun looking for just that. A loose alliance of companies including Gilead, Novartis, Schrödinger, Takeda Pharmaceutical, and WuXi AppTec has formed to share ideas, resources, and data with the goal of developing custom pan-coronavirus antivirals. They say it’s a philanthropic, not a commercial, effort and that the tools they discover along the way will be put in the public domain.
Researchers in the alliance are working at a speed and level of openness they compare to a wartime effort. And while they hope their collaboration could shave years off the time it takes to discover an antiviral, they stress that none of their work is likely to reach the clinic in time to address this pandemic.
“That’s a tall order for us to be able to impact short term,” says Jay Bradner, president of the Novartis Institutes for BioMedical Research (NIBR). But given the global death toll and the disruption pervading daily life, the companies say they are committed to continuing the initiative beyond the current crisis. “While it may not help the COVID-19 pandemic, we feel responsible to deliver a bespoke coronavirus therapy,” Bradner says.
A like-minded team
The effort began with a phone call in mid-March between five R&D leaders, including Bradner and Andrew Plump, president of R&D at Takeda. All had been dealing with the ramifications of the coronavirus outbreak at their companies’ sites in China, and the depth of its effect on the US and Europe was starting to become clear. Cases of COVID-19, the respiratory illness caused by the novel coronavirus, were growing exponentially.
“The reality is that while we’re competitors, we’re also long-standing colleagues in this industry, and many of us are close friends,” Plump says. “We all started to say, ‘Listen, let’s just get on the phone and figure out how we can help each other.’ ”
The gatherings began as a way to “talk through these playbooks we were initially writing on the fly,” Bradner recalls. The early conversations were logistical, largely focused on matters like when to institute work from home and how to sustain vital research amid shutdowns. “Along the way, these dialogues became intensely scientific,” Bradner says. “R&D leaders started mapping out what it would look like to organize around repurposing science.”
The group grew and combined with a loose assembly of venture capital executives. Calling itself COVID R&D, the larger group had a broad mandate: vaccines, antibody treatments, drug repurposing, and novel antivirals.
“We kind of signed a pact in blood that we weren’t going to make this bureaucratic,” Plump says. Who discovered what was less important than the “need to come together and make things happen,” he adds.
Out of COVID R&D emerged a smaller team that Steve Hitchcock, Takeda’s head of research, describes as “like-minded research organizations” working together on antiviral discovery. It includes Novartis, which is focused on antivirals that target SARS-CoV-2’s main protease; Takeda, which is working on antivirals against several targets, is sharing a library of protease inhibitors plus structural and computational biology expertise; Gilead, which is exploring RNA polymerase inhibitors; WuXi, which is providing assays and chemistry; and Schrödinger, which is providing computational screens of target proteins as well as a data-sharing platform.
“We all came together,” Hitchcock says, “and started to discuss: What are our strengths, and how could we put together a virtual drug discovery pipeline by drawing from the strengths of each one of these individual organizations and recognize what we can do?”
Blocking the coronavirus
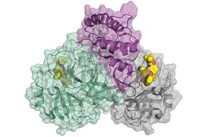
Novartis had already been quietly exploring how to design inhibitors of the coronavirus’s main protease, known as MPro.
When the genetic sequences of the various SARS-CoV-2 viral proteins became available, they revealed remarkable similarities between its main protease and those of two coronaviruses responsible for earlier outbreaks. “The overlay of the main protease from SARS-CoV-2 to SARS to MERS is chilling,” Bradner says, referring to the severe acute and Middle East respiratory syndromes.
This realization planted a seed of hope. If a drug could block that protein, it stood a good chance of working against not just this coronavirus but whatever one emerged next.
The main protease had another intriguing feature: snapshots revealed a cysteine dangling in its active site—the pocket where partner molecules dock to turn it on and off. When the coronavirus translates its genome, it churns out a long string of peptides that the main protease snips down to size. “This enzyme is really well behaved and has at the center of it this amino acid—a cysteine—that coordinates the chemistry,” says Daniel Nomura, a chemical biologist at the University of California, Berkeley, and the Innovative Genomics Institute.
Nomura’s lab, along with several others at UC Berkeley, has spent the past 3 years collaborating with Novartis on ways to find druggable pockets on proteins using cysteine-reactive probes.
That work meant that Novartis and Berkeley researchers had on hand an array of tools to develop covalent inhibitors that tether to cysteines on proteins, including MPro. “We have this very large library of cysteine-targeting covalent ligands that we’ve been building out over many years,” Nomura says. “We thought that was a perfect way into targeting the catalytic cysteine of what I would consider a highly druggable protein.”
A plus is that covalent inhibitors should look more like traditional small-molecule drugs than the peptide-based compounds typically used to inhibit proteases. Those drugs have proved difficult to develop into pills.
The groundwork from the Novartis-Berkeley collaboration has significantly accelerated the search for pancoronavirus inhibitors. Nomura says a package containing a tiny tube filled with the MPro gene arrived at his labs during the week of March 16. A week later, scientists at Novartis had used that gene to express large quantities of the protein, which they passed back to the Berkeley researchers. By March 30, initial screens of their cysteine-targeting ligands against the protein turned up several hits.
“This has been one of the fastest-moving projects I’ve ever been a part of,” Nomura says. Until mid-March, he didn’t know anything about coronaviruses. Since then, with the six scientists from the labs of Nomura and F. Dean Toste, and working with scientists across three Novartis sites, “we’ve been able to generate really potent inhibitors against the coronavirus main protease and even solve structures of them.”
“You really want to inhibit completely this protease,” he says. “In fact, our best inhibitor totally wipes out activity in the enzyme.” Importantly, those initial compounds are able to bind to and shut down activity of not only SARS-CoV-2 but also the coronaviruses responsible for SARS and MERS.
Nomura usually works on some of the toughest drug targets—messy proteins that lack well-defined pockets. “It makes me think I should have gone after easier targets to start with,” he jokes.
A coalition forms
Bradner presented the early stages of Novartis’s work with Berkeley on one of the COVID R&D phone calls and mentioned that if companies had any protease inhibitors in their vaults, Novartis’ scientists would be willing to screen them against the assays they’d developed for the coronavirus main protease he says. “Andy Plump from Takeda was the first to throw up his hand.”
Plump says he had been struck by Bradner’s description of similarities between the main protease of SARS-CoV-2 and those of the viruses that cause SARS and MERS. Companies briefly mobilized at the time of the SARS outbreak but let those efforts wither when the outbreak waned. “If we had continued with SARS activities, we might actually have a therapy that wouldn’t be a repurposed therapy but a specific therapy to target [SARS-]CoV-2,” Plump says.
Researchers at Takeda, which since early on during the coronavirus outbreak was publicly pursuing hyperimmune antibodies to treat COVID-19, had more quietly been thinking about antiviral drug discovery. Like most big drug firms these days, the company lacks active virology or infectious disease programs. But Takeda does have an extensive library of proteases—spanning some 15,000 compounds, including some covalent binders, Hitchcock says—and other compound libraries that could prove useful for viral targets.
Takeda began its own internal efforts on several viral proteins, and also brought to the antiviral alliance two additional partners that are providing critical resources. The contract research firm WuXi is providing screening capabilities and chemists, while Schrödinger is complementing lab work with computational screens.
“When they came to us, we didn’t say yes right away,” Schrödinger CEO Ramy Farid says. The company had been approached by many organizations wanting to use Schrödinger’s platform as part of their response to the outbreak.

Although Schrödinger has a long-standing collaboration with Takeda, Farid wanted to know more about the targets the alliance was considering. His concern was that computational methods aren’t very useful without good structural information on the viral proteins.
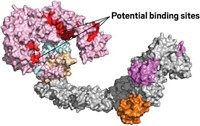
Before signing on, Schrödinger analyzed the 16 proteins that together are responsible for viral replication. Its scientists found that most lacked structures, and the ones that did exist weren’t very good. But high-resolution structures have been captured for two, and possibly three, proteins, and there are even a few snapshots of molecules bound to their active sites, Farid says. It was enough to give him the confidence that Schrödinger could help.
Schrödinger added another resource to the mix: a way for companies to share information. The company uses a platform called LiveDesign that allows humans and computers to collaborate on molecular design ideas. As Farid describes it, the platform generates billions of ideas around many different scaffolds, then filters those down to a manageable number of molecules—say, 10–20—that a chemist might want to make.
After scientists synthesize those compounds and test them for their ability to stop the virus in the lab, they enter the data into the computer, which uses the information to generate a new set of molecules to test. “And that’s all done in real time so that everybody has access to the database,” Farid says.
That process is already underway. Takeda researchers, which have access to Schrodinger’s software, conducted a virtual screen of the main protease to winnow their vast compound libraries down to a more focused library of 10,000 compounds. That was shipped to WuXi for screening in its protein enzymatic assay, Hitchcock says. These main-protease inhibitors complement the covalent inhibitors Novartis is developing. An agreement allows Takeda to transfer compounds to Novartis to test its own assays.
Hitchcock says Takeda is now going through the same type of exercise with Gilead, but with a focus on RNA polymerase—the target of Gilead’s remdesivir. Gilead did not respond to interview requests.
Advertisement
Open-ended science
All this work is happening at a pace and with a transparency that defy industry norms. Hitchcock says that in Zoom meetings, researchers may not even know who works for what company. While the compounds being developed are encouraging, everyone cautions that none of them will be ready for human testing in time for this pandemic.
The industry scientists are also acutely aware that drug firms tend to lose interest in infectious disease projects when a pathogenic threat fades. They claim that one prerequisite for participating in the alliance—aside from openness with data and the understanding that the effort is philanthropic—is a commitment to keep plugging away at pan-coronavirus inhibitors after society starts to reopen.
Takeda, for one, says it will commit to “a 2-year-plus program to bring a molecule forward,” Plump says.
And even as they pool resources, the executives acknowledge that their capabilities are not comprehensive. “One thing we really need to put in place is downstream virology support and animal model support,” says John Tallarico, NIBR’s head of chemical biology and therapeutics. Novartis is discussing whether the right strategy is working with academic collaborators, contract research firms, or both, he notes.
Novartis is “perfectly comfortable” putting the assays, reagents, and protocols it develops into the public domain, Bradner says. “What could be better for humanity than the race for main-protease inhibitors to become competitive?”
That sounds like good public relations, but Bradner insists that’s not the aim. “This isn’t altruism. This is ruthlessly strategic.” The overarching goal, he says, is for the scientific community to come up with a solution to this—or the next—pandemic.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter