Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Discovery
Amazing Women
Michelle Lynn Hall
Modeling mastermind is using computational chemistry to help scientists craft mRNA therapies
by Ryan Cross
August 25, 2019
| A version of this story appeared in
Volume 97, Issue 33

Credit: Courtesy of Michelle Lynn Hall/Moderna/Shutterstock/C&EN

COVER STORY
Michelle Lynn Hall
Vitals
Current affiliation: Moderna
Age: 35
PhD alma mater: Columbia University
If I weren’t a chemist, I would be: “A marine biologist. I’m an avid scuba diver who will eagerly jump in the water with just about any animal.”
I’ve overcome adversity in the lab by: “Prior to joining Moderna, and like too many other women in science, I was frequently sexually harassed. It took me a long time to get the courage to speak up, fearing serious retaliation and irreparable damage to my professional reputation and career. Unfortunately, my experience is far from unique, and many talented people leave science due to sexual harassment and other forms of discrimination. This has to end, and I hope that I can play a role in changing our field for the better by speaking out.”
Must-have in the lab: “My ‘lab’ is a laptop connected to cloud-computing instances, but my go-to is still old-school pen and paper. I’ll scribble just about anything anywhere and still have my coveted idea notebooks dating all the way back to undergrad.”
Must-have on the road: “I have a special fondness for narwhals. Neither unicorns nor mermaids are real, but somehow the mermaid unicorn (i.e., the narwhal) is?! I have a small plush narwhal that I pack with me on trips to remind me to always question what we ‘know’ to be ‘true.’ ”
3 key papers
“Automated Protocol for Large-Scale Modeling of Gene Expression Data” (J. Chem. Inf. Model. 2016, DOI: 10.1021/acs.jcim.6b00260)
“Automated Ligand- and Structure-Based Protocol for In Silico Prediction of Human Serum Albumin Binding” (J. Chem. Inf. Model. 2013, DOI: 10.1021/ci3006098)
“Quantitative DFT Modeling of the Enantiomeric Excess for Dioxirane-Catalyzed Epoxidations” (J. Am. Chem. Soc. 2009, DOI: 10.1021/ja806951r)
When Michelle Lynn Hall was looking for a new job in 2016, Moderna wasn’t an obvious first choice. Hall, a computational chemist, was well versed in quantum mechanical simulations and atomic-level molecular dynamics—techniques useful in small-molecule and protein-based drug discovery.
Moderna had plans to do something very different: make drugs out of messenger RNA, the intermediate molecule between our genes and our proteins. The Cambridge, Massachusetts–based company often describes mRNA as software for cells, a molecular instruction manual for making therapeutic proteins that fight infections, combat cancer, and treat rare diseases. “It sounded like science fiction,” Hall says. “And that was the draw.”
But making mRNA therapy a reality is not so straightforward. mRNA is a large, unwieldy molecule. Predicting how its thousands of nucleotide building blocks will interact with different parts of cells—such as the machines that translate the mRNA code or the immune sentinels that spot and destroy foreign mRNA—is a headache. To help solve that problem, Iain McFadyen, Moderna’s head of computational sciences, wanted to hire the company’s first computational chemist. He needed someone undaunted by uncharted territory, and Hall fit the bill.
As a graduate student, Hall made a last-minute decision to join a computational chemistry lab even though she arrived at Columbia University intent on studying organic chemistry. When Hall’s postdoc adviser at Novartis gave her a list of potential projects to work on, she picked one that she knew nothing about. And as a researcher at Schrödinger, a chemical software company, Hall used machine learning to analyze gene expression data for drug discovery—a task well outside the firm’s expertise.
Hall hardly knew a thing about mRNA when McFadyen interviewed her, but he was impressed with her experience and fearlessness. “She was the only one I considered for the position,” he says.
Hall immediately set out to model mRNA’s structure and function, and her work has helped Moderna’s scientists design better molecules. In the process, she learned about another, even bigger challenge that could make or break mRNA therapy’s ability to live up to its full potential: the delivery dilemma.
To get mRNA into cells of the body, Moderna scientists were packaging it in fatty spheres called lipid nanoparticles. Lipid nanoparticles aren’t new. Other companies have used them to deliver small interfering RNAs, short nucleotide strands that can block the production of problematic proteins. The lack of safe and effective nanoparticles almost derailed the development of those therapies. Moderna doesn’t want the same thing to happen to mRNA therapy, and it needs new nanoparticles that can hold the comparatively giant mRNA molecules.
Each of Moderna’s lipid nanoparticles is a collection of about 60,000 lipid and cholesterol molecules. But scientists still don’t understand how those molecules coalesce into a structure that encapsulates mRNA. Hall thought that a computational model could help answer that question.
At Moderna’s annual science day in May, Hall presented the first results of that project. She used an approach called coarse-grained molecular dynamics to study how those thousands of nanoparticle parts interact. She discovered that the first 1.3 µs of the reaction are crucial for encapsulating mRNA.
Those insights will help Moderna’s lab chemists create new recipes and protocols for making nanoparticles that better package and deliver their mRNA cargo. The firm is still years away from getting its first drug approved—and there’s no guarantee that it will happen. But if it does, Hall will likely have played a vital role in transforming mRNA therapy from the realm of science fiction to reality.
When Michelle Lynn Hall was looking for a new job in 2016, Moderna wasn’t an obvious first choice. Hall, a computational chemist, was well versed in quantum mechanical simulations and atomic-level molecular dynamics—techniques useful in small-molecule and protein-based drug discovery.
Moderna had plans to do something very different: make drugs out of messenger RNA, the intermediate molecule between our genes and our proteins. The Cambridge, Massachusetts–based company often describes mRNA as software for cells, a molecular instruction manual for making therapeutic proteins that fight infections, combat cancer, and treat rare diseases. “It sounded like science fiction,” Hall says. “And that was the draw.”
Advertisement
But making mRNA therapy a reality is not so straightforward. mRNA is a large, unwieldy molecule. Predicting how its thousands of nucleotide building blocks will interact with different parts of cells—such as the machines that translate the mRNA code or the immune sentinels that spot and destroy foreign mRNA—is a headache. To help solve that problem, Iain McFadyen, Moderna’s head of computational sciences, wanted to hire the company’s first computational chemist. He needed someone undaunted by uncharted territory, and Hall fit the bill.
As a graduate student, Hall made a last-minute decision to join a computational chemistry lab even though she arrived at Columbia University intent on studying organic chemistry. When Hall’s postdoc adviser at Novartis gave her a list of potential projects to work on, she picked one that she knew nothing about. And as a researcher at Schrödinger, a chemical software company, Hall used machine learning to analyze gene expression data for drug discovery—a task well outside the firm’s expertise.
Hall hardly knew a thing about mRNA when McFadyen interviewed her, but he was impressed with her experience and fearlessness. “She was the only one I considered for the position,” he says.
Hall immediately set out to model mRNA’s structure and function, and her work has helped Moderna’s scientists design better molecules. In the process, she learned about another, even bigger challenge that could make or break mRNA therapy’s ability to live up to its full potential: the delivery dilemma.
To get mRNA into cells of the body, Moderna scientists were packaging it in fatty spheres called lipid nanoparticles. Lipid nanoparticles aren’t new. Other companies have used them to deliver small interfering RNAs, short nucleotide strands that can block the production of problematic proteins. The lack of safe and effective nanoparticles almost derailed the development of those therapies. Moderna doesn’t want the same thing to happen to mRNA therapy, and it needs new nanoparticles that can hold the comparatively giant mRNA molecules.
Each of Moderna’s lipid nanoparticles is a collection of about 60,000 lipid and cholesterol molecules. But scientists still don’t understand how those molecules coalesce into a structure that encapsulates mRNA. Hall thought that a computational model could help answer that question.
At Moderna’s annual science day in May, Hall presented the first results of that project. She used an approach called coarse-grained molecular dynamics to study how those thousands of nanoparticle parts interact. She discovered that the first 1.3 µs of the reaction are crucial for encapsulating mRNA.
Those insights will help Moderna’s lab chemists create new recipes and protocols for making nanoparticles that better package and deliver their mRNA cargo. The firm is still years away from getting its first drug approved—and there’s no guarantee that it will happen. But if it does, Hall will likely have played a vital role in transforming mRNA therapy from the realm of science fiction to reality.
Vitals
Current affiliation: Moderna
Age: 35
PhD alma mater: Columbia University
If I weren’t a chemist, I would be: “A marine biologist. I’m an avid scuba diver who will eagerly jump in the water with just about any animal.”
I’ve overcome adversity in the lab by: “Prior to joining Moderna, and like too many other women in science, I was frequently sexually harassed. It took me a long time to get the courage to speak up, fearing serious retaliation and irreparable damage to my professional reputation and career. Unfortunately, my experience is far from unique, and many talented people leave science due to sexual harassment and other forms of discrimination. This has to end, and I hope that I can play a role in changing our field for the better by speaking out.”
Must-have in the lab: “My ‘lab’ is a laptop connected to cloud-computing instances, but my go-to is still old-school pen and paper. I’ll scribble just about anything anywhere and still have my coveted idea notebooks dating all the way back to undergrad.”
Must-have on the road: “I have a special fondness for narwhals. Neither unicorns nor mermaids are real, but somehow the mermaid unicorn (i.e., the narwhal) is?! I have a small plush narwhal that I pack with me on trips to remind me to always question what we ‘know’ to be ‘true.’ ”
Research at a glance
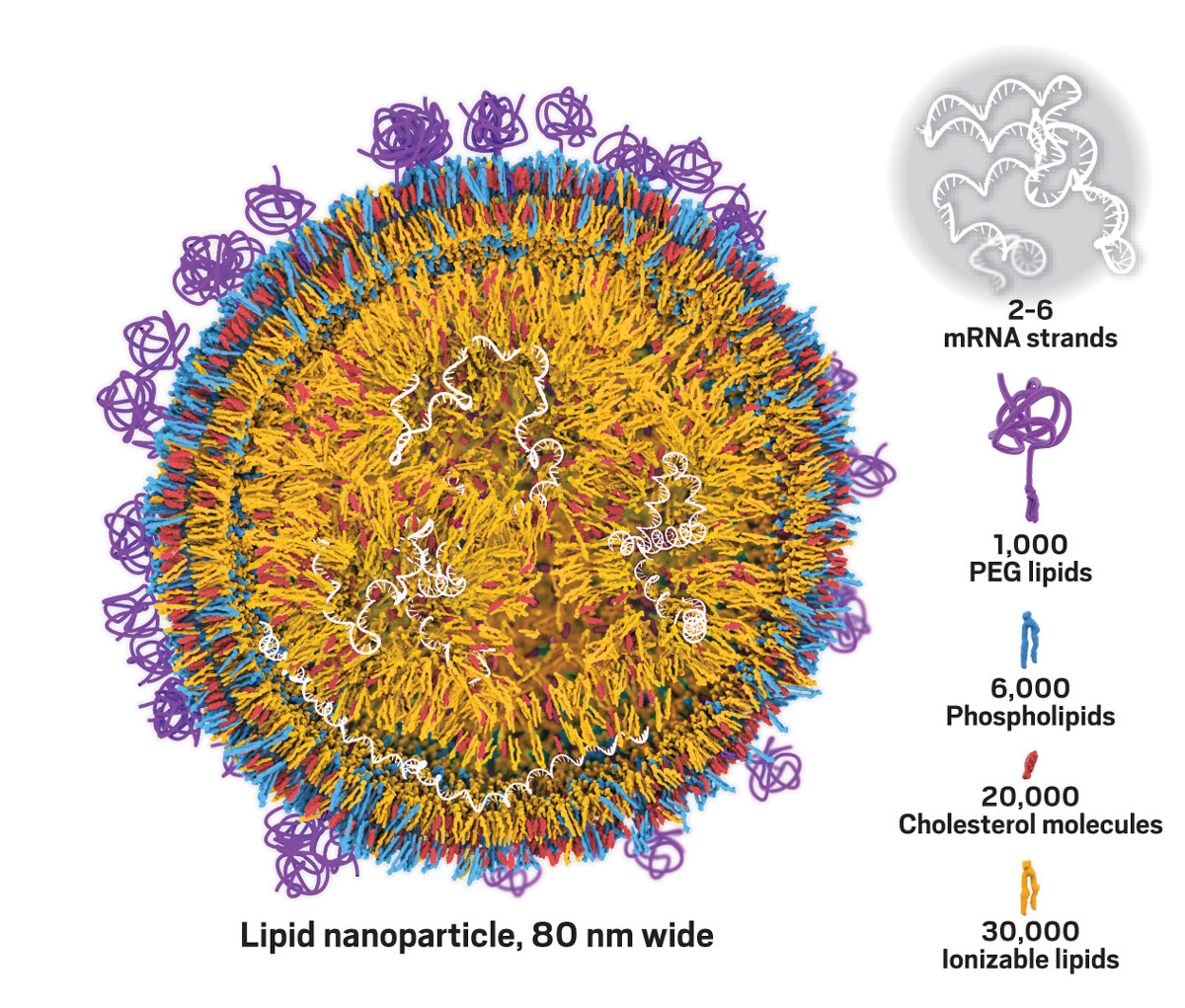
Credit: Moderna/C&EN
Hall studies how the building blocks of lipid nanoparticles encapsulate mRNA therapies. She leads a project that uses computer models to simulate how nearly 60,000 molecules coalesce into a particle.
Three key papers
“Automated Protocol for Large-Scale Modeling of Gene Expression Data”
(J. Chem. Inf. Model. 2016, DOI: 10.1021/acs.jcim.6b00260)



















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter