Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Computational Chemistry
Infinitene might be aromatic
Calculations suggest that the twisted molecule reported in 2021 has cylindrical aromaticity
by Laura Howes
April 7, 2022
| A version of this story appeared in
Volume 100, Issue 12
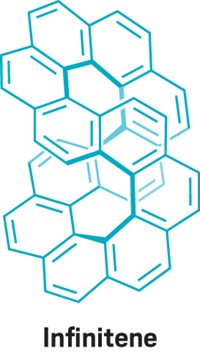
The infinity symbol–shaped molecule known as infinitene caught chemists’ attention in 2021. Calculations from a team at the University of Helsinki now suggest that the molecule might be aromatic after all (Phys. Chem. Chem. Phys. 2022, DOI: 10.1039/d2cp00637e).
Last year, a team at Nagoya University reported the successful synthesis of a looped benzocirculene made of 12 benzene rings fused into the shape of an infinity symbol or figure eight. C&EN readers voted infinitene the 2021 Molecule of the year (J. Am. Chem. Soc. 2022, DOI: 10.1021/jacs.1c10807).
When the Nagoya team reported their achievement, they calculated that the aromaticity was confined to individual rings rather than spread over the whole molecule. But Mesías Orozco-Ic, Rashid R. Valiev, and Dage Sundholm decided to run their own analysis. The shape of the new molecule is quite a challenge for calculating aromaticity. For example, its twisted form means that simple electron counting rules such as Hückel or Möbius aromaticity rules are not valid.
The Helsinki team used a quantum chemistry software package, Turbomole, to simulate infinitene’s response to an external magnetic field. Their calculations indicate that in a magnetic field, delocalized electrons would flow along the two edges of the molecule in two nonintersecting paths, a characteristic of aromatic molecules. As chemists synthesize more twisted structures, these sorts of calculations might uncover aromatic molecules that simple electron-counting rules miss.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter