
PACKING THE RIGHT
MOLECULES
FOR THE
GREAT
OUTDOORS
Cutting-edge chemistry makes recreation in the wild more enjoyable—and more comfortable.
Illustration by Alexander Wells
Funke has spent most of his life outdoors and, over the years, has witnessed many improvements in the gear he uses, such as new garment and tent textiles and ultralight solar chargers. Chemistry drives many of these technologies.
Solar-powered safety
Some outdoor enthusiasts enjoy a digital detox, but many keep their devices powered up for safety. Leading groups on guided hikes in the mountains, Funke knows the routes—but he also knows the importance of digital trekking maps and global positioning systems. “If you get into bad weather, you can lose your orientation really quickly,” he says.
USB sockets may not be found in nature, but the best source of energy is there for the taking: the sun. Organic photovoltaic (OPV) solar cells are a relatively new photovoltaic (PV) technology. All PVs convert photons of light into electricity and rely on an electron acceptor, such as a fullerene, to accept the photons. OPVs use an organic molecular or polymeric absorber to collect photons from sunlight.
The organic absorber and acceptor materials in OPVs are ultrathin and lightweight, making them ideal for outdoor recreation. Danish company InfinityPV manufactures OPV cells that are printed onto flexible foil just 100 μm thick. Their compact solar charger rolls up into a tube and can be stuffed into a backpack. When a hiker or climber needs to power up a device, she can unroll the charger, lay it out on any surface—such as a rock, slope, or pathway—and let it absorb sunlight.
Funke has noticed the changes this new technology has brought. “Sometimes people take a small tablet with solar cells,” he says. “They can email; they can go on the internet almost anywhere. It's really changed.”
Snug and dry
When Funke leads hikes in the Sierra Nevada, he contends with elemental extremes. “You set off in shorts and T-shirts,” he says, “but when you reach Mulhacén, the highest peak, it's very windy.” He says there are now materials, light and comfortable to wear, that offer very effective protection from cold, wind, rain, and snow than older textiles.
Fabrics, coatings, and textile treatments have helped us stay warm and dry throughout history, and chemistry is the key. Traditional oilskins were made from old ship sails coated in linseed oil and wax. And the famous Mackintosh raincoat, patented in 1823, came about when inventor Charles Macintosh bound together two pieces of rubber by dissolving them in naphtha, a volatile liquid hydrocarbon mixture.
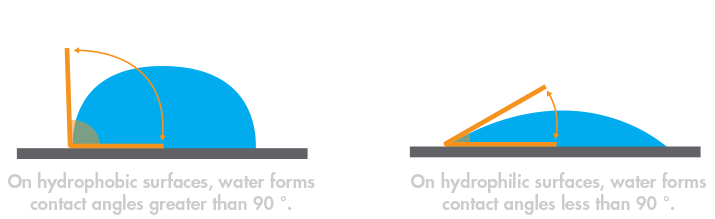

The next revolution in outdoor clothing took more than another century to arrive. In 1969, Wilbert L. Gore and his son Robert W. exploited the qualities of polytetrafluoroethylene (PTFE), commonly known as Teflon™, to produce a breathable, waterproof membrane. PTFE repels water because it forms a hydrophobic surface that causes water to bead up and drop off. The breathable, waterproof membrane is based on expanded polytetrafluoroethylene (ePTFE), an ultrathin membrane that contains over 9 billion pores per square inch, typically layered between nylon and polyurethane for durability and extra protection. Water vapor from sweat can pass through the membrane, but liquid water cannot.
Despite advances in synthetic materials, nature still inspires innovation. Colmar, the Italian ski-and-activewear company, uses Teflon EcoElite™, a plant-based, nonfluorinated fabric treatment for water repellency, to produce high-performance garments for outdoor recreation. The finish is sourced from 60% renewable raw materials.
Clothes aren't the only textiles that have changed for the better. Modern tents tend to be constructed with lightweight nylon fibers coated with silicone or urethane to aid waterproofing. Classic A-frame tents have been superseded by domes and tunnels, which are more stable and easier to set up. Low weight and high packability are also essential. Lightweight poles made of aluminum alloys or carbon fibers help manufacturers produce ultralight shelters; some shed more weight by allowing hikers to use their trekking poles to set up instead.
Hot stuff
When Funke leads an overnight hike, he carries dry food, such as pasta. But if people want to stay longer they need to carry something even lighter. “They need these ‘astronaut’ foods because other types are just too heavy,” Funke says.
What Funke calls “astronaut” foods are lightweight, self-heating culinary packs. They can be cooked anywhere, without a heat source, and eliminate the need for heavy stoves and cookware. The military largely gets the credit for this particular bit of chemical wizardry.
The Meal, Ready to Eat (MRE) is the primary individual ration of the U.S. Armed Forces. “The majority of the current MRE components are either thermostabilized, water-activity controlled, or extruded. They are all packaged in foil pouches,” explains Michelle Richardson of the Food Engineering and Analysis Team at the Combat Feeding Directorate, U.S. Army.
Thermostabilization uses a combination of pressure and temperature to destroy bacterial spores and make the meal palatable. MREs can be eaten cold, but soldiers—like nearly everyone else—prefer a warm meal, especially when operating in cold, wet conditions.
A flameless ration heater, or FRH, is a water-activated exothermic chemical heater that can raise the temperature of a 226.8-gram MRE by 56 °C in 10 to 12 minutes without a visible flame.

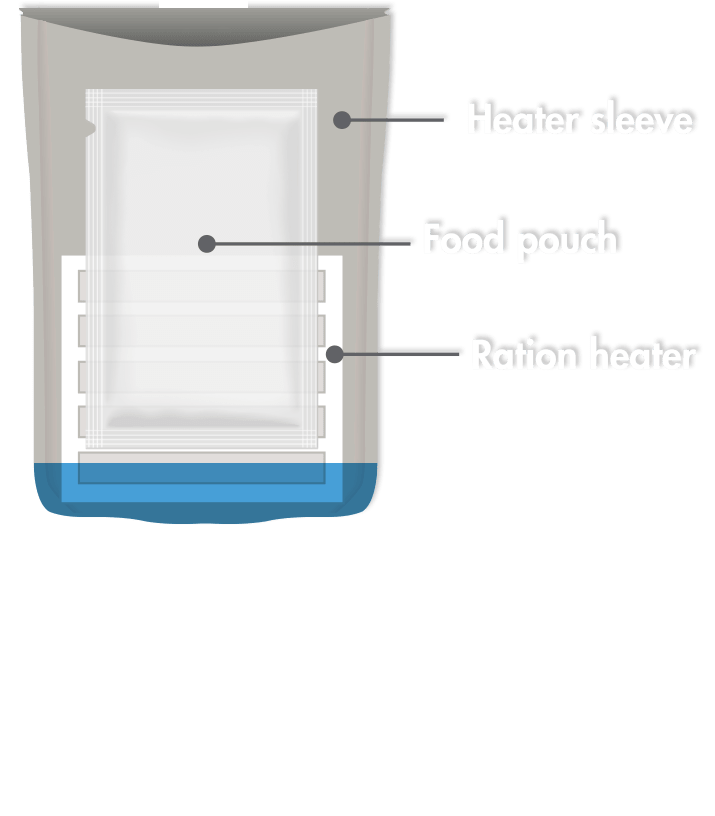
FRHs generate heat in an oxidation-reduction reaction. FRHs contain finely powdered magnesium metal, alloyed with a small amount of iron and sodium chloride. When a hungry diner adds water to the reaction heater, the water triggers the oxidation-reduction reaction and the water quickly reaches its boiling point.

Iron metal and chloride ions accelerate the reaction, but don't appear to significantly contribute to heat production. They act more like catalysts; chloride results in MgOCl formation that helps water molecules penetrate the passivating MgO layer, and iron accelerates the formation of hydrogen gas.
“The ration heater contains finely powdered magnesium metal, alloyed with a small amount of iron and sodium chloride,” explains Lauren Oleksyk, team leader of the Food Engineering and Analysis Team. “To activate the reaction, a small amount of water is added, which quickly reaches the boiling point.”
It is no accident that outdoor enthusiasts reap the benefits of military invention. The Combat Feeding Directorate works with industry and academia under Dual Use Science and Technology programs to ensure that new food-processing technologies, such as preservation and packaging systems, can be spun off into the civilian marketplace.
Call of nature
No matter how people cook their food, they'll need to deal with what comes next.
Many trailheads and outdoor recreation sites now provide facilities, the simplest form of which is a hole in the ground. Flushable chemical toilets feature basic plumbing systems and tanks that contain chemicals, such as formaldehyde or, increasingly, digestive enzymes, to neutralize odors and disinfect waste.
Those looking for an eco-friendly answer to nature's call can go with composting toilets, ideal for remote locations. They require little water or energy, depending instead on aerobic organisms to decompose human waste. They work, first, by employing gravity, which naturally separates solids and urine (urea and ammonia). Bacteria then break down the solids by up to 90% and convert the urine into nitrogen-rich liquids that can be used as fertilizer. These clever little organisms also kill harmful pathogens.
Human waste disposal is challenging enough in the wild, but it's a public health crisis in inhabited areas with little to no sewerage infrastructure. In fact, it's one of the most pressing challenges of the 21st century. “Currently 4.5 billon people lack access to adequate sanitation,” explains Cody Finke, a member of the California Institute of Technology team that won the Bill & Melinda Gates Foundation's Reinvent the Toilet challenge in 2012.
Finke and colleagues have engineered a modular wastewater treatment system for use in places without sewers. Their solution, which runs on solar power, breaks down human waste in a biodigester and an electrochemical reactor connected in series. The system recycles water for flushing and even operates at a surplus, since water goes in every time someone urinates.
The waste in the chemical toilet developed by Cody Finke’s team is sent to an anaerobic bioreactor. The organic matter breaks down to release gases, such as methane and carbon dioxide.
Effluent from the bioreactor is then sent to an electrochemical reactor. In the reactor, sodium chloride and water are mixed to produce hypochlorous acid, sodium hydroxide, and hydrogen gas.
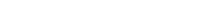
Several reactions occur in the electrochemical reactor. Oxidation of chloride (Cl–) to Cl2 occurs at the anode first.

Then Cl2 reacts with water to form hypochlorous acid at the reactor’s anode. This reaction is in equilibrium. Depending on the water quality, the reaction of hypochlorous acid with organics may lead to the production of byproducts that need to be removed.

Hydrogen gas appears at the cathode by taking electrons from sodium chloride and protons from water.
Sodium hydroxide is produced at the cathode. In the process, harmful micropollutants, organic matter, and pathogens are degraded or removed.
Tough textiles
When it comes to comfort and safety in the outdoors, material construction is as important as coatings and treatments.
In some ways, Funke says, little has changed for climbers over the past 20 years. “In the end, it's still about you and the rock. You have to use your technique and your force,” he says. But the so-called dynamic ropes that climbers use have certainly improved. “They are thinner and lighter these days,” he says. “They are easier to carry and handle.”

Unlike static ropes, dynamic ropes are slightly stretchy, meaning they can absorb the shock of a sudden fall without snapping—a quality that has no doubt saved many climbers' lives. Dynamic ropes consist of a core of thousands of braided polymer fibers (usually nylon) wrapped in a tough outer layer, or sheath, of smoother nylon strands.
Nylon 6 is formed through a ring-opening polymerization. A small amount of initiator, which can be water or a strong base (water is used in the example below), is added to the cyclic caprolactam starting material.
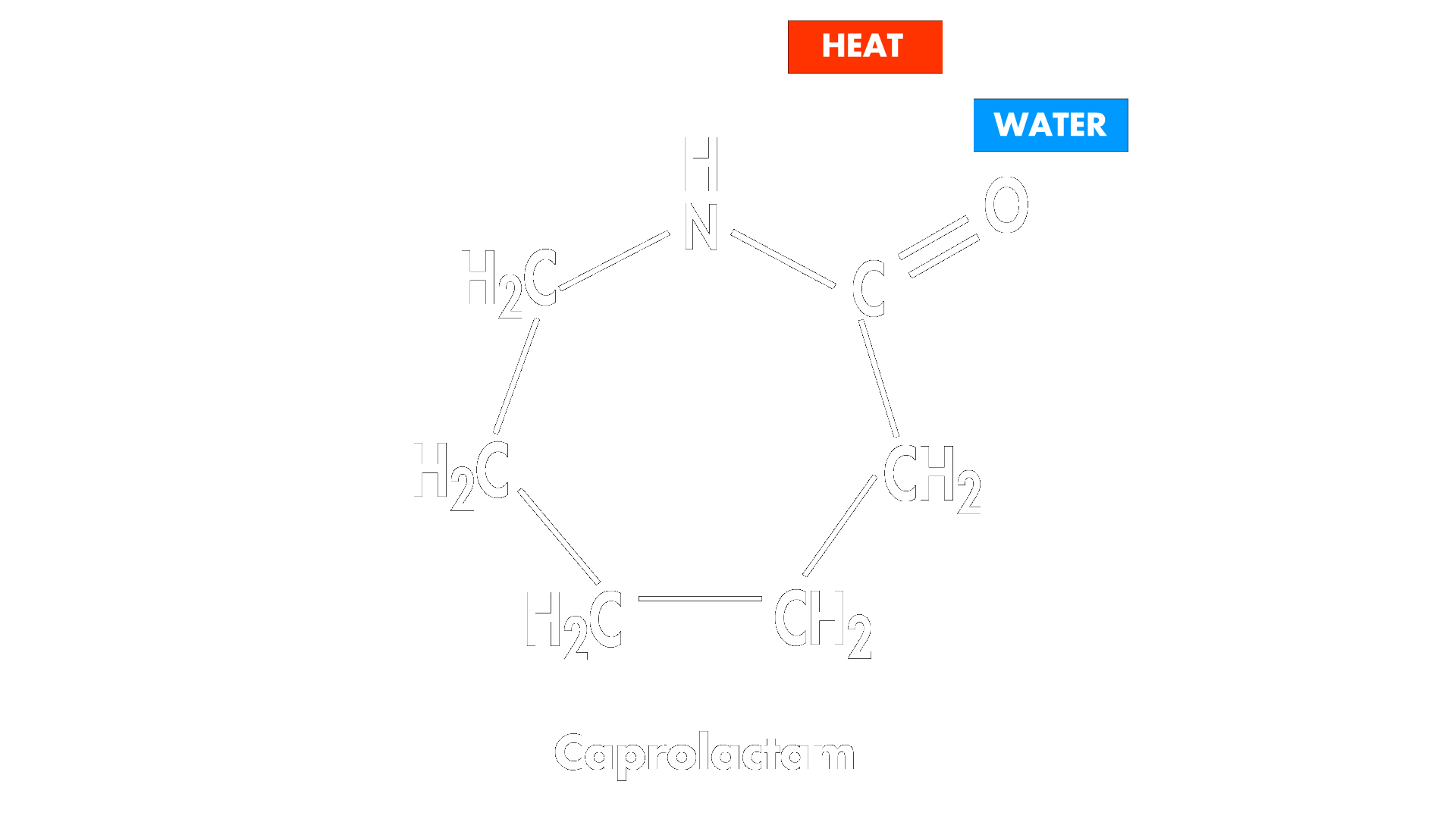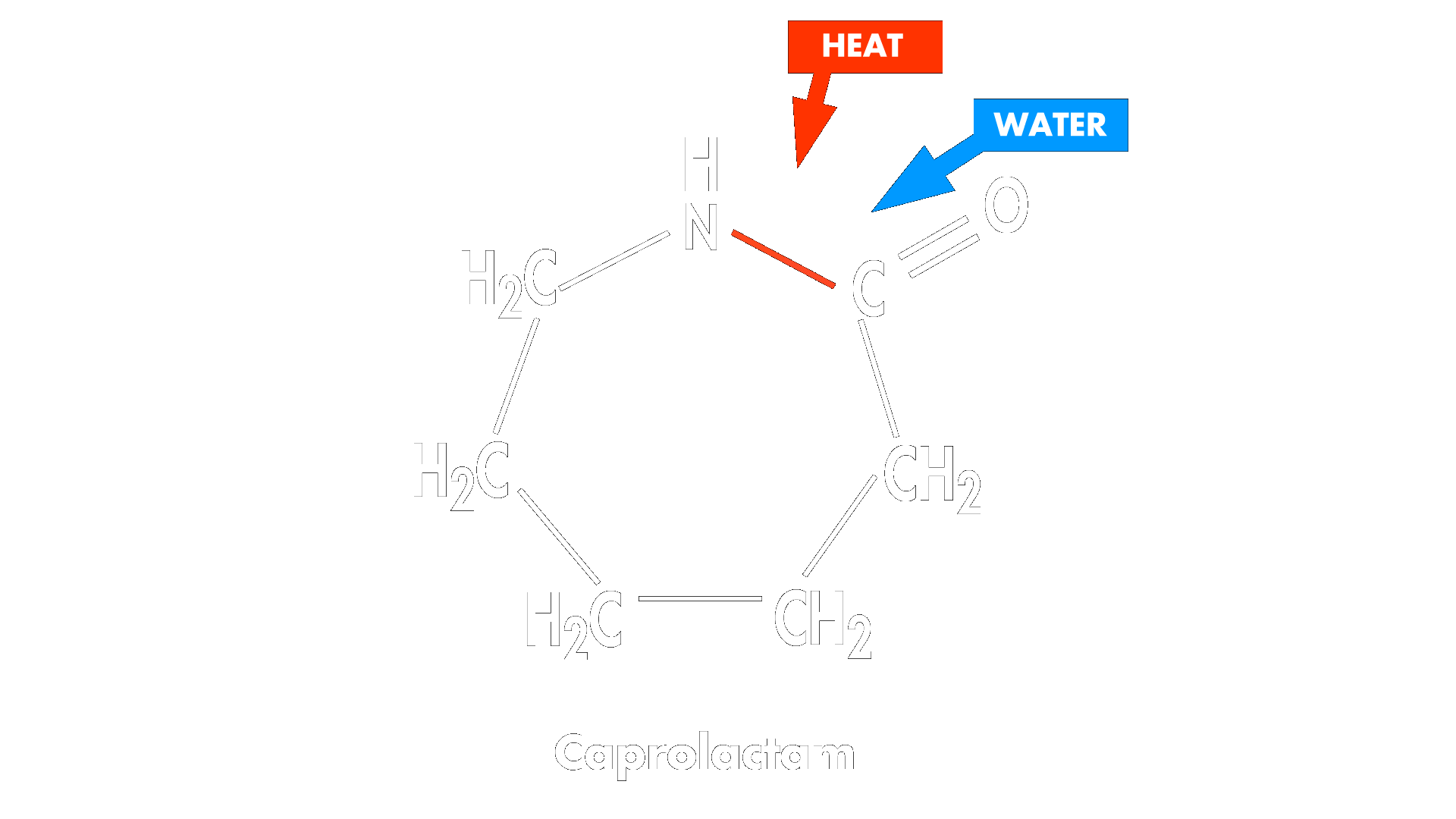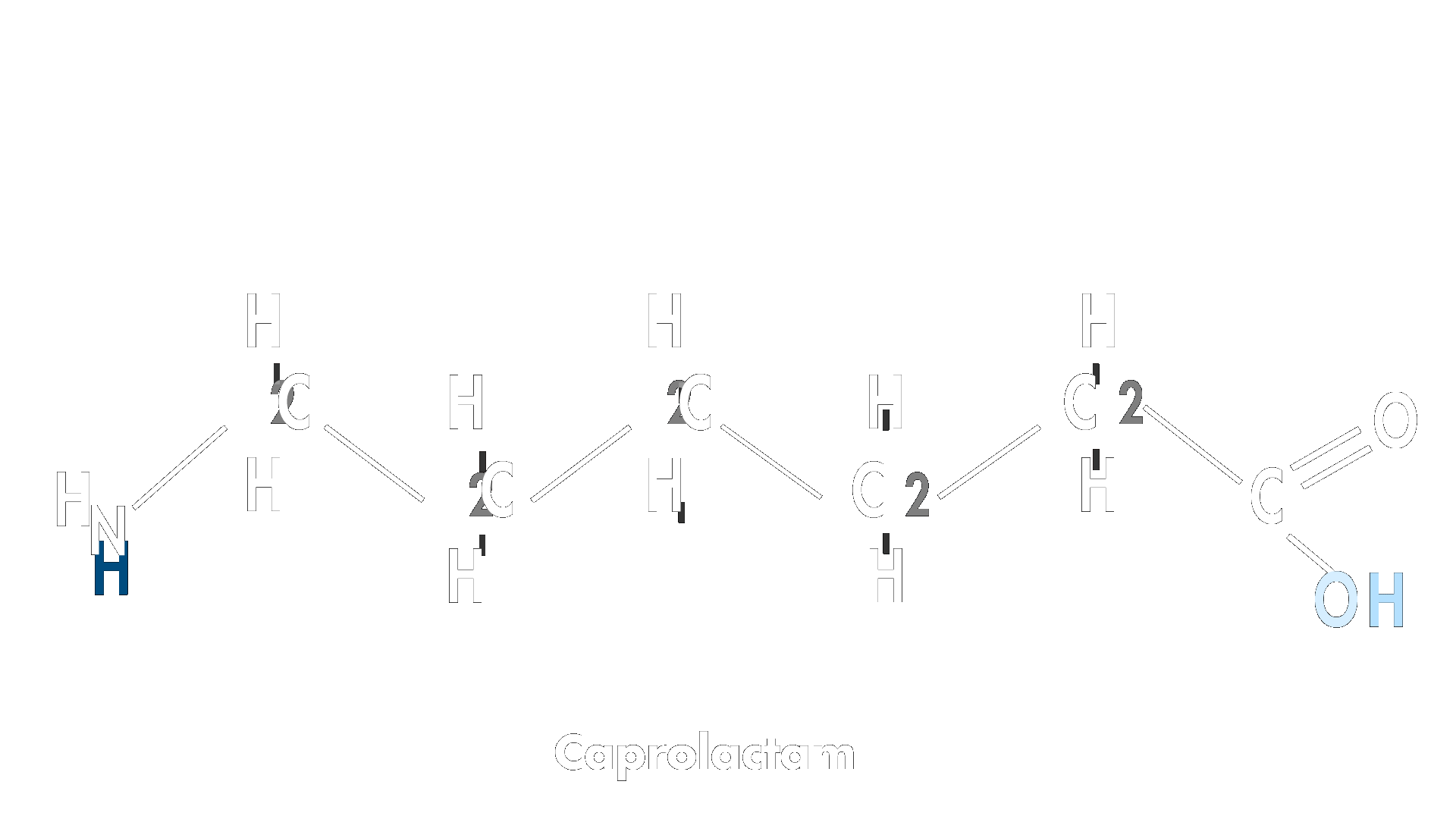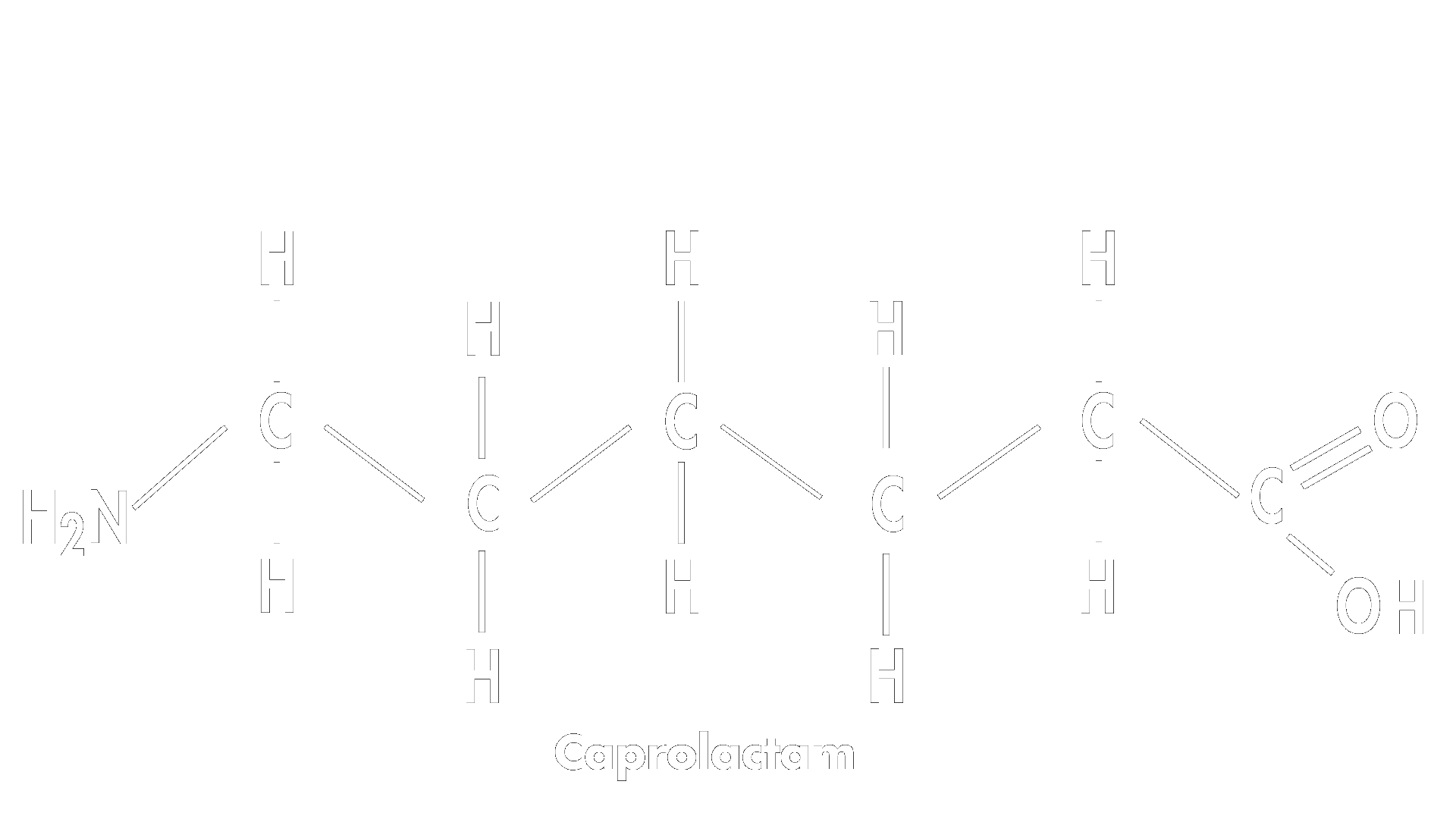
This linear intermediate attacks the carbonyl carbon of another molecule of caprolactam to extend the chain and the reaction continues to form long polymer chains.

Nylon 6,6 is formed through a condensation reaction, in which the nitrogen atoms in hexamethylene diamine attack the carbonyl carbons of adipic acid and displace the OH groups. Water is formed as a byproduct. Stringing these molecules together forms the long polymer chains that are used as the raw material to make the rope.

Another textile construction technique, downproofing, ensures that the down (feathers) or, alternatively, synthetic fibers in sleeping bags stay in place, helping keep outdoor sleepers dry and warm. A typical downproof nylon may have a total count of 320 threads per square inch, with 190 threads on the warp loom and a fill of 130 threads, explains Gary Peterson, managing director at Western Mountaineering, a sleeping bag manufacturer. “Our thread counts tend to be over 400 in most cases and they are all either perfectly balanced or very nearly square in terms of the number of yarns in the warp and fill directions. This leads to a more balanced fabric that is tighter, more downproof, stable and also more water resistant.
Embrace the great outdoors
Even after all these years, Funke still marvels at the beauty of Tarifa's rugged coastline and its cork and olive tree-covered hills. Sure, the gear may have changed, but he still sees the same scenes at day's end: Tired hikers peel off garments, campers snuggle up in their sleeping bags, and climbers pack up their ropes. Only now, the clothes are waterproof, the sleeping bags are downproofed, the ropes are dynamic, and there's a new hue to the nightly diorama: the glow of smartphone screens charged up from stored solar energy.
New chemistry continues to make getting out into nature easier and more comfortable. But there is one major improvement Funke would still like to see. It's not from chemistry, though; it's from us.
“There are so many adventure sports,” he says. “Enjoy life in the outdoors more.”
Precipitating the conversation:
A Perspective from Thomas Band, Fluoropolymers Business Manager at the Chemours Company

Respect for nature. That's a principle that many outdoor enthusiasts—myself included—adopt whenever they venture into wild areas. Minimizing human impact helps maintain the integrity of ecosystems and preserves our natural heritage. Those of us who work in the chemistry industry want to extend that philosophy as much as we can to the gear we take with us to the trail. Fortunately, chemical innovations like Teflon EcoElite™ finish for textiles make that possible.
Getting out into nature wasn’t always as easy on us as it is now. Heavy gear with inadequate weatherproofing and waterproofing often left outdoor enthusiasts exhausted, soaked, and chilled. But technical fabrics came along to combat the elements, creating lightweight tents, packs, boots, and apparel that repel moisture.
Performance Meets Sustainability
Now we're unlocking new chemistries that allow us to combine high performance with high sustainability. Nothing exemplifies our holistic view of sustainability more than Teflon EcoElite™. Not only does it provide better runnability, moisture resistance, and durable repellency compared to other products, it does so with durable, non-GMO, renewable sources. It's the only product on the market with that robust mix of performance and sustainability.
I believe caring for the natural world should extend beyond my time in the wild, and I’m glad to be working at a chemistry company that’s innovating to make that possible. New chemistries are providing remarkable performance with increased sustainability.
Teflon EcoElite™ is doing more than just helping us brave and protect the wild. It's even begun to improve our everyday clothes, making them more resistant to the elements. Chemours is working with the textile industry to improve the stain-fighting ability of our carpets and linens and to keep those in our homes and cars looking better longer.
For now, though, I’m happy that Teflon EcoElite™ is helping preserve the natural world. The more of us there are experiencing natural splendor, the more of us there are who will be passionate about protecting it.
READ ABOUT HOW CHEMOURS HELPS BRING COMFORT TO WORKOUTS:
August 20: The Chemistry of Amusement Parks
October 1: The Chemistry of Sports
November 26: The Chemistry of Travel
December 17: The Chemistry of Dining
HOW CHEMISTRY ROCKS MUSIC FESTIVALS
ABOUT SPONSORED CONTENT
Sponsored content is not written by and does not necessarily reflect the views of C&EN's editorial staff. It is authored by writers approved by the C&EN BrandLab and held to C&EN's editorial standards, with the intent of providing valuable information to C&EN readers. This sponsored content feature has been produced with funding support from The Chemours Company.




