Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Catalysis
Same catalyst, different times, give different enantiomers
Reaction forms chiral amines in high selectivity
by Leigh Krietsch Boerner
July 6, 2020
| A version of this story appeared in
Volume 98, Issue 26

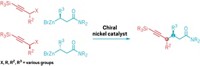
Different molecular enantiomers can have different chemistry, both in the body and in industrial processes. Controlling the yield of the right one is a time-consuming yet critical step in organic and pharmaceutical synthesis. Shu-Li You and coworkers at the Shanghai Institute of Organic Chemistry have found a surprising and simple shortcut: they can selectively make either enantiomer of some chiral amines just by varying the reaction times (Nat. Chem. 2020, DOI: 10.1038/s41557-020-0489-1). They used an iridium cyclooctadiene compound and a chiral olefin to create a chiral catalyst in solution, a common approach for asymmetric synthesis. After 6 min, S isomers form with 84–99% enantiomeric purity. If the researchers let the reaction go for 10 h, the R isomer forms with 74–99% enantiomeric purity (shown). The catalyst is highly selective for the S isomer and makes the compound quickly, You says. Over time, however, it decomposes and the more stable R isomer forms. The team discovered this while monitoring the reaction every 10 min. You says he was shocked by the results, as no one has reported this effect in asymmetric catalysis before. The team is now studying if the effect occurs with other reactions.



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter