Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.

Medicinal Chemistry
A century of medicinal molecules
Chemists’ work creating lifesaving drugs lies at the heart of modern medicine. These 5 drugs are among those that have changed the practice of medicine, the science of drug discovery, and the very essence of society over the past 100 years
by Bethany Halford , Alla Katsnelson, special to C&EN
August 11, 2023
| A version of this story appeared in
Volume 101, Issue 26
Penicillin
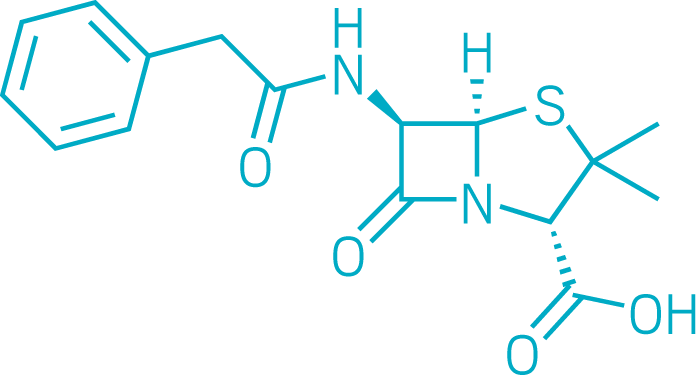
Penicillin, one of the first antibiotics, has saved millions of lives. It enabled physicians to treat simple infections that had often been fatal.
Alexander Fleming famously discovered the drug in a moldy petri dish in 1928. Eleven years later, Howard Florey and Ernst Chain at the University of Oxford set out to confirm penicillin’s bacteria-killing properties in mice and to test it in people. In 1941, a police constable from the city of Oxford became the first person to be successfully treated with penicillin, though he ultimately died because supplies of the antibiotic ran out.
With World War II unfolding, British scientists reached out to US colleagues to scale up production. Penicillin was first used to treat infections in wounded Allied soldiers; the wonder drug was severely rationed for civilians. But by the mid-1940s, large-scale fermentation methods were in place at commercial production facilities.
Penicillin remains one of the most widely used antibiotics in the world, although many others have been developed. As more bacteria develop antibiotic resistance, new approaches to antibiotic discovery are urgently needed.
Chlorpromazine
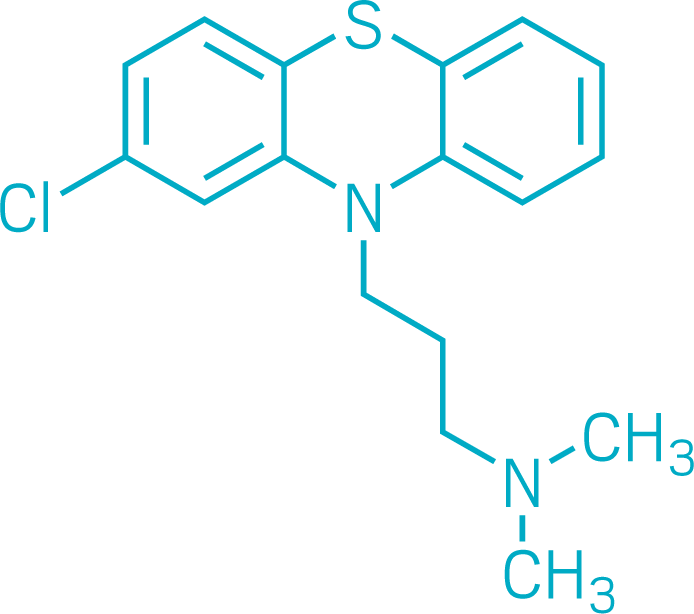
Chlorpromazine was one of the first drugs to directly treat psychiatric illness. When chemists at the French pharmaceutical firm Rhône-Poulenc first synthesized it in December 1950, treatments for psychotic disorders like schizophrenia were ineffective and often dangerous.
But chlorpromazine wasn’t developed as a psychiatric drug. At the time, French physician Henri Laborit was testing whether antihistamines, which treat allergies, nausea, and insomnia, could keep patients calm while under surgical anesthesia. In January 1952, he persuaded colleagues to give chlorpromazine, along with several other drugs, to a 24-year-old patient experiencing psychosis. After 3 weeks of the therapy, the man was well enough to leave the hospital.
The use of chlorpromazine in people with psychosis spread quickly around the world. Smith, Kline & French bought the right to sell the drug—marketed as Thorazine—in North America. Approved by the US Food and Drug Administration in 1954, chlorpromazine became one of the most successful new drugs, earning the company $10 million within a year, C&EN reported soon after.
Chlorpromazine remains in use today, although newer antipsychotics are available. Its success demonstrated that mental illness could be treated chemically, ushering in the age of psychopharmacology and changing the practice of psychiatry forever.
The birth control pill
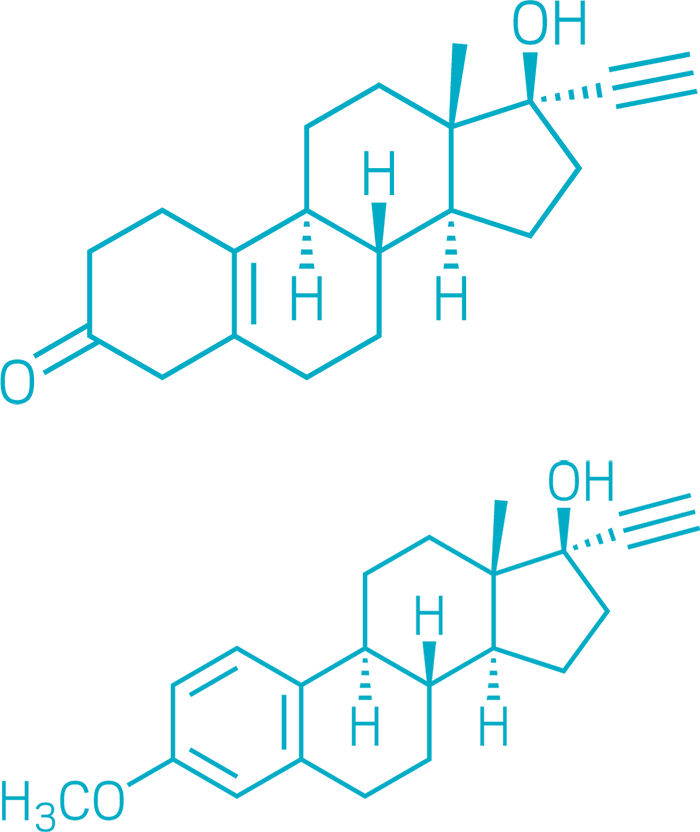
Norethynodrel (Top), Mestranol (Bottom)
Oral birth control is so influential that it bears the simplest of pharmaceutical nicknames: the pill. Activists Margaret Sanger and Katharine McCormick, biologist Gregory Pincus, and gynecologist John Rock are often credited with the development of hormonal birth control. But chemists made the pill a reality.
By the 1920s, scientists knew that giving reproductive hormones like progesterone to animals prevents pregnancy. But this strategy was impractical as a medicine: progesterone had to be either injected or taken orally in enormous doses.
Working for Syntex in 1951, chemists Carl Djerassi, Luis Miramontes, and George Rosenkranz synthesized norethindrone—the first progesterone analog that was effective at low doses when taken orally. A year later, Frank B. Colton, a chemist at G.D. Searle, synthesized a similar compound, norethynodrel.
The norethynodrel used in early clinical trials was contaminated with mestranol, an analog of the hormone estradiol and a by-product of norethynodrel synthesis. Pure norethynodrel turned out to induce bleeding between menstrual periods, so mestranol was added to the first oral contraceptive formulations.
The US Food and Drug Administration approved Enovid, a combination of norethynodrel and mestranol, in 1957 to treat menstrual disorders and in 1960 as an oral contraceptive—the first in the US. This year, the agency made one version of the pill available over the counter. By giving women the power of family planning, oral birth control revolutionized society.
Antiretroviral therapy

(Top to bottom) Tenofovir alafenamide, Emtricitibine, Dolutegravir
The molecules pictured here—tenofovir alafenamide, emtricitibine, and dolutegravir—are just 3 of more than 30 drugs used in different combinations for antiretroviral therapy (ART) to treat HIV. ART has changed the infection from a death sentence to a manageable chronic condition.
ART owes its success to myriad researchers in academia, government, and industry. In 1987, the US Food and Drug Administration approved zidovudine, marketed as AZT. The drug inhibited the virus’s reverse transcriptase and prevented it from making copies of itself. But the compound had toxic side effects, and the virus quickly developed resistance to it.
Scientists realized that to rein in HIV, they needed to target multiple stages of the virus’s replication cycle. In 1995, the FDA approved saquinavir, the first protease inhibitor for HIV, and the following year, doctors reported that administering multiple drugs from different classes chased the virus into remission. Between 1996 and 1999, death rates from HIV in the US and Europe fell by more than 50%.
More classes of ART drugs have emerged since, and more recent versions have fewer side effects. Doctors in the US now prescribe ART as soon as someone is diagnosed with HIV, to reduce not only mortality and morbidity but also the chance that the virus will be passed on. People at high risk of infection can take these drugs preventively.
Imatinib
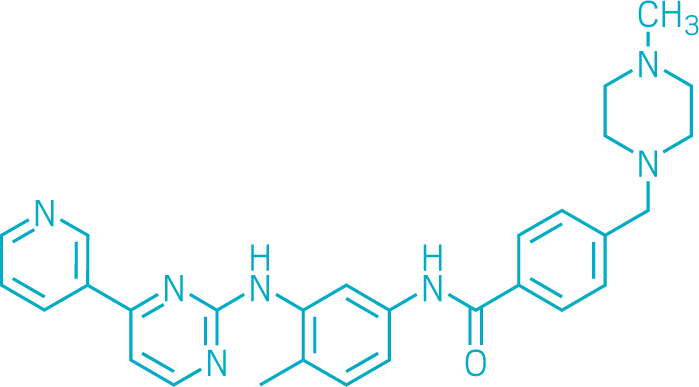
Imatinib was one of the first drugs designed to counteract a specific cancer-causing mutation in a gene. Its success helped establish the age of precision medicine. First approved by the US Food and Drug Administration in 2001 and sold under the name Gleevec in the US, imatinib proved to be a highly effective treatment for chronic myeloid leukemia (CML). It raised the 5-year survival rate for people with this cancer from 30% to about 90%.
Brian Druker, at the Oregon Health and Science University, and biochemist Nicholas Lyndon, then at pharmaceutical firm Ciba-Geigy (now Novartis), invented imatinib in the late 1990s. The drug blocks the ATP-binding site of a tyrosine kinase that signals cancer cells to multiply. The kinase is produced in people with CML by a mutated gene created by a DNA swap between two chromosomes.
Drugmakers had previously shied away from blocking kinases. The body contains so many that they doubted a drug could differentiate between useful and harmful versions. But imatinib did the job. The drug is now used to treat several cancers, and there are dozens of other cancer medicines that target specific mutations or whose design was otherwise informed by genomics.





Penicillin

Penicillin, one of the first antibiotics, has saved millions of lives. It enabled physicians to treat simple infections that had often been fatal.
Alexander Fleming famously discovered the drug in a moldy petri dish in 1928. Eleven years later, Howard Florey and Ernst Chain at the University of Oxford set out to confirm penicillin’s bacteria-killing properties in mice and to test it in people. In 1941, a police constable from the city of Oxford became the first person to be successfully treated with penicillin, though he ultimately died because supplies of the antibiotic ran out.
With World War II unfolding, British scientists reached out to US colleagues to scale up production. Penicillin was first used to treat infections in wounded Allied soldiers; the wonder drug was severely rationed for civilians. But by the mid-1940s, large-scale fermentation methods were in place at commercial production facilities.
Penicillin remains one of the most widely used antibiotics in the world, although many others have been developed. As more bacteria develop antibiotic resistance, new approaches to antibiotic discovery are urgently needed.
Chlorpromazine

Chlorpromazine was one of the first drugs to directly treat psychiatric illness. When chemists at the French pharmaceutical firm Rhône-Poulenc first synthesized it in December 1950, treatments for psychotic disorders like schizophrenia were ineffective and often dangerous.
But chlorpromazine wasn’t developed as a psychiatric drug. At the time, French physician Henri Laborit was testing whether antihistamines, which treat allergies, nausea, and insomnia, could keep patients calm while under surgical anesthesia. In January 1952, he persuaded colleagues to give chlorpromazine, along with several other drugs, to a 24-year-old patient experiencing psychosis. After 3 weeks of the therapy, the man was well enough to leave the hospital.
The use of chlorpromazine in people with psychosis spread quickly around the world. Smith, Kline & French bought the right to sell the drug—marketed as Thorazine—in North America. Approved by the US Food and Drug Administration in 1954, chlorpromazine became one of the most successful new drugs, earning the company $10 million within a year, C&EN reported soon after.
Chlorpromazine remains in use today, although newer antipsychotics are available. Its success demonstrated that mental illness could be treated chemically, ushering in the age of psychopharmacology and changing the practice of psychiatry forever.
The birth control pill

Oral birth control is so influential that it bears the simplest of pharmaceutical nicknames: the pill. Activists Margaret Sanger and Katharine McCormick, biologist Gregory Pincus, and gynecologist John Rock are often credited with the development of hormonal birth control. But chemists made the pill a reality.
By the 1920s, scientists knew that giving reproductive hormones like progesterone to animals prevents pregnancy. But this strategy was impractical as a medicine: progesterone had to be either injected or taken orally in enormous doses.
Working for Syntex in 1951, chemists Carl Djerassi, Luis Miramontes, and George Rosenkranz synthesized norethindrone—the first progesterone analog that was effective at low doses when taken orally. A year later, Frank B. Colton, a chemist at G.D. Searle, synthesized a similar compound, norethynodrel.
The norethynodrel used in early clinical trials was contaminated with mestranol, an analog of the hormone estradiol and a by-product of norethynodrel synthesis. Pure norethynodrel turned out to induce bleeding between menstrual periods, so mestranol was added to the first oral contraceptive formulations.
The US Food and Drug Administration approved Enovid, a combination of norethynodrel and mestranol, in 1957 to treat menstrual disorders and in 1960 as an oral contraceptive—the first in the US. This year, the agency made one version of the pill available over the counter. By giving women the power of family planning, oral birth control revolutionized society.
Antiretroviral therapy

The molecules pictured here—tenofovir alafenamide, emtricitibine, and dolutegravir—are just 3 of more than 30 drugs used in different combinations for antiretroviral therapy (ART) to treat HIV. ART has changed the infection from a death sentence to a manageable chronic condition.
ART owes its success to myriad researchers in academia, government, and industry. In 1987, the US Food and Drug Administration approved zidovudine, marketed as AZT. The drug inhibited the virus’s reverse transcriptase and prevented it from making copies of itself. But the compound had toxic side effects, and the virus quickly developed resistance to it.
Scientists realized that to rein in HIV, they needed to target multiple stages of the virus’s replication cycle. In 1995, the FDA approved saquinavir, the first protease inhibitor for HIV, and the following year, doctors reported that administering multiple drugs from different classes chased the virus into remission. Between 1996 and 1999, death rates from HIV in the US and Europe fell by more than 50%.
More classes of ART drugs have emerged since, and more recent versions have fewer side effects. Doctors in the US now prescribe ART as soon as someone is diagnosed with HIV, to reduce not only mortality and morbidity but also the chance that the virus will be passed on. People at high risk of infection can take these drugs preventively.
Imatinib

Imatinib was one of the first drugs designed to counteract a specific cancer-causing mutation in a gene. Its success helped establish the age of precision medicine. First approved by the US Food and Drug Administration in 2001 and sold under the name Gleevec in the US, imatinib proved to be a highly effective treatment for chronic myeloid leukemia (CML). It raised the 5-year survival rate for people with this cancer from 30% to about 90%.
Brian Druker, at the Oregon Health and Science University, and biochemist Nicholas Lyndon, then at pharmaceutical firm Ciba-Geigy (now Novartis), invented imatinib in the late 1990s. The drug blocks the ATP-binding site of a tyrosine kinase that signals cancer cells to multiply. The kinase is produced in people with CML by a mutated gene created by a DNA swap between two chromosomes.
Drugmakers had previously shied away from blocking kinases. The body contains so many that they doubted a drug could differentiate between useful and harmful versions. But imatinib did the job. The drug is now used to treat several cancers, and there are dozens of other cancer medicines that target specific mutations or whose design was otherwise informed by genomics.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter