Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Support nonprofit science journalism
C&EN has made this story and all of its coverage of the coronavirus epidemic freely available during the outbreak to keep the public informed. To support us:
Donate Join Subscribe

A year in the COVID-19 pandemic
From the discovery of SARS-CoV-2 to the rollout of the first vaccines, here are the key moments in scientists’ effort to fight the coronavirus
January 25, 2021 | Appeared in Volume 99, Issue 3
December 31, 2019
Pandemic milestones

The Wuhan Municipal Health Commission reports 27 unusual cases of pneumonia.
January 11, 2020
Pandemic milestones
China reports the first death from the novel coronavirus.
January 13, 2020
Vaccines
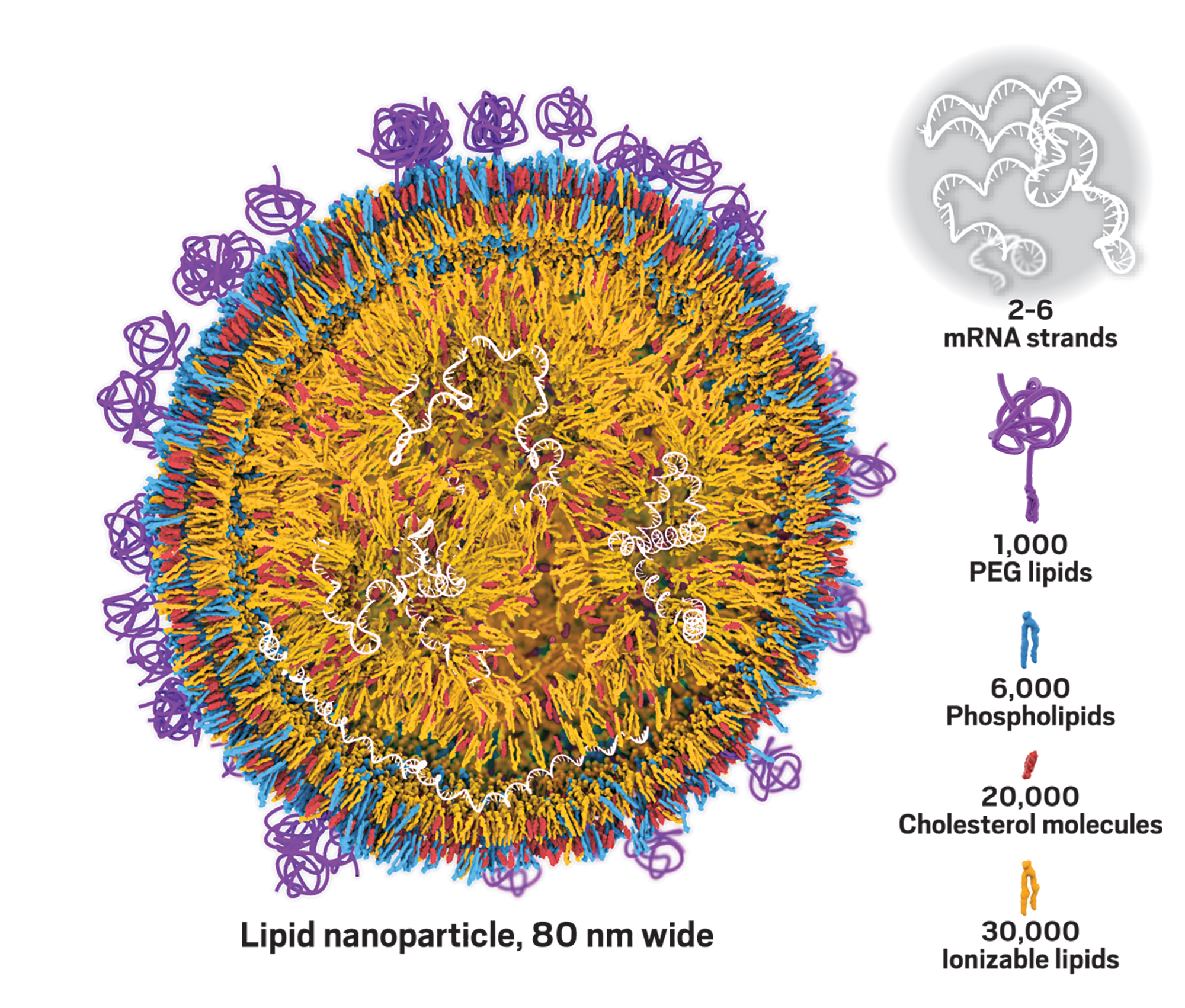
Moderna announces plans to develop and test a messenger RNA (mRNA) vaccine against the novel coronavirus. Read more in C&EN.
January 13, 2020
Pandemic milestones
The first lab-confirmed infection with the coronavirus outside China is reported.
January 21, 2020
Pandemic milestones
The US Centers for Disease Control and Prevention reports the first coronavirus case in the US, a patient in Washington State.
January 30, 2020
Pandemic milestones
The World Health Organization (WHO) declares the coronavirus a public health emergency of international concern. Read more in C&EN.
February 11, 2020
Pandemic milestones
The International Committee on Taxonomy of Viruses officially names the novel coronavirus “severe acute respiratory syndrome coronavirus 2” (SARS-CoV-2), and the WHO names the disease it causes "COVID-19" (for "coronavirus disease 2019").
March 11, 2020
Pandemic milestones
The WHO declares the coronavirus outbreak a pandemic.
March 16, 2020
Vaccines
CanSino Biologics and Moderna both start Phase 1 clinical trials of COVID-19 vaccines. Read more in C&EN.
March 18, 2020
Drugs
The WHO launches the Solidarity trial, a multiarm, global study to compare the efficacy of four drugs or drug combinations.
March 21, 2020
Diagnostics
The US Food and Drug Administration grants an emergency use authorization (EUA) for the first COVID-19 point-of-care diagnostic.
March 23, 2020
Drugs
The UK government launches RECOVERY (Randomised Evaluation of COVID-19 Therapy), a large, randomized clinical trial of several repurposed and new treatments for COVID-19. Read more in C&EN.
March 27, 2020
Pandemic milestones
US Congress passes the Coronavirus Aid, Relief, and Economic Security (CARES) Act, providing $2.2 trillion in economic stimulus. Read more in C&EN.
March 27, 2020
Pandemic milestones

British prime minister Boris Johnson becomes the first world leader to contract COVID-19.
March 27, 2020
Diagnostics
Abbott Laboratories receives an EUA for its rapid SARS-CoV-2 RT-PCR test.
March 28, 2020
Drugs
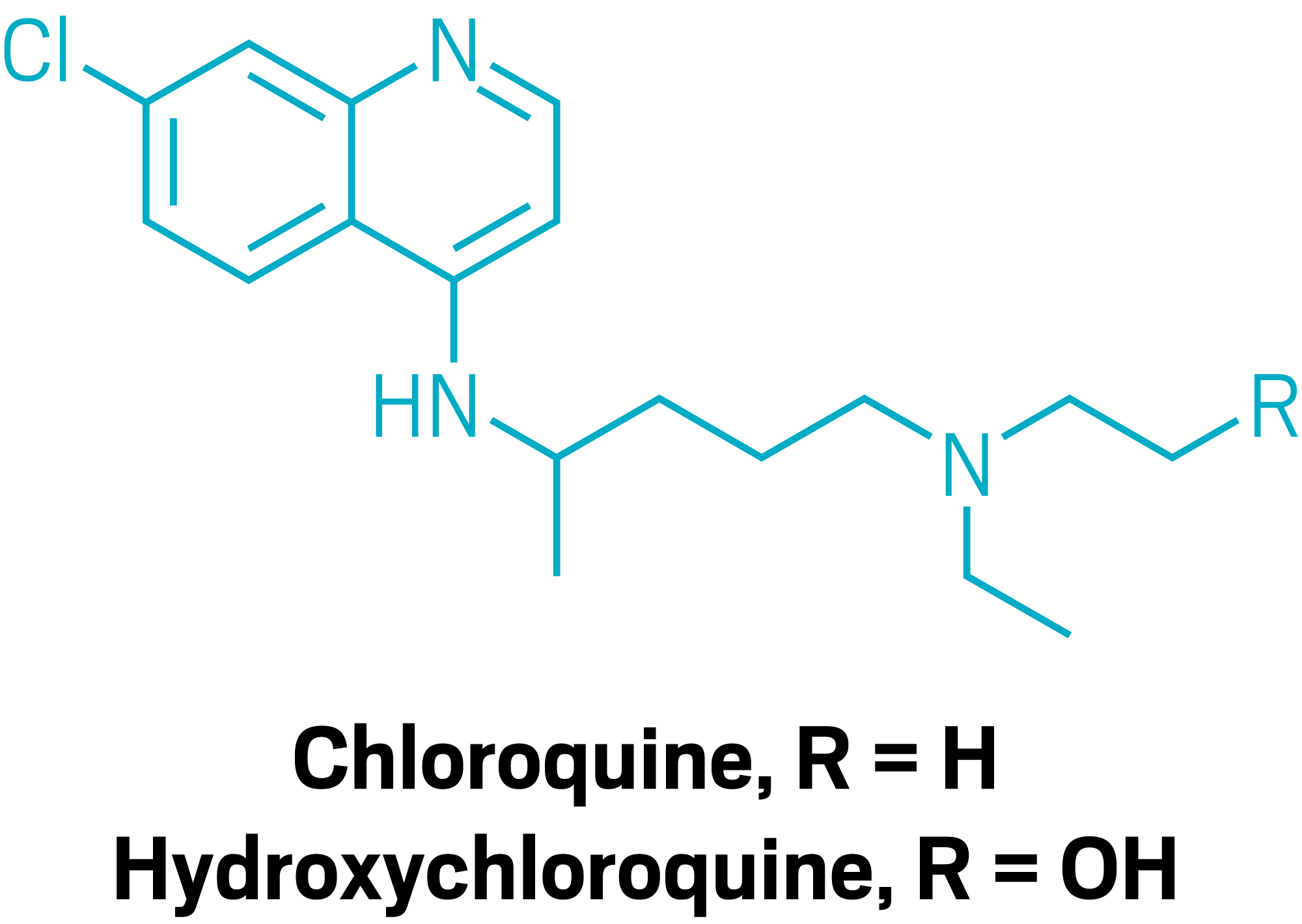
The FDA grants an EUA for the antimalarials chloroquine and hydroxychloroquine. Read more in C&EN.
April 4, 2020
Pandemic milestones
The WHO reports over 1 million cases of COVID-19 have been confirmed worldwide.
April 10, 2020
Pandemic milestone
More than 100,000 people worldwide have died from the novel coronavirus.
April 21, 2020
Diagnostics
The FDA authorizes the first COVID-19 diagnostic that allows a sample to be collected at home.
April 23, 2020
Vaccines
University of Oxford researchers begin a Phase 1 trial of their adenoviral vector vaccine, which is later licensed to AstraZeneca.
May 5, 2020
Vaccines
The first participant in the US is dosed in the Phase 1 trial of Pfizer and BioNTech's mRNA vaccine.
May 7, 2020
Diagnostics

Sherlock Biosciences receives an EUA for the first CRISPR diagnostic for COVID-19.
May 9, 2020
Diagnostics
The FDA grants an EUA for the first antigen test for rapid detection of the virus. Read more in C&EN.
May 15, 2020
Pandemic milestone
US president Donald J. Trump announces that Operation Warp Speed will supply 300 million vaccines to the US by January 2021.
May 18, 2020
Vaccines
Moderna announces preliminary Phase 1 data from its vaccine trial.
May 22, 2020
Vaccines
CanSino publishes the first peer-reviewed data of its Phase 1 vaccine trial for its adenoviral vector vaccine.
May 28, 2020
Pandemic milestones
The US death toll crosses 100,000.
May 29, 2020
Vaccines
Moderna doses the first volunteers in its Phase 2 trial of its mRNA vaccine.
June 1, 2020
Drugs
The first drug designed specifically for COVID-19, a monoclonal antibody from Eli Lilly and Company that targets the SARS-CoV-2 spike protein, begins human studies.
June 11, 2020
Drugs
Regeneron Pharmaceuticals begins Phase 1 studies of a monoclonal antibody cocktail that targets the SARS-CoV-2 spike protein.
June 15, 2020
Drugs
The FDA withdraws the EUA for the antimalarials chloroquine and hydroxychloroquine and recommends against using the drugs in combination with the antiviral remdesivir.
June 16, 2020
Drugs
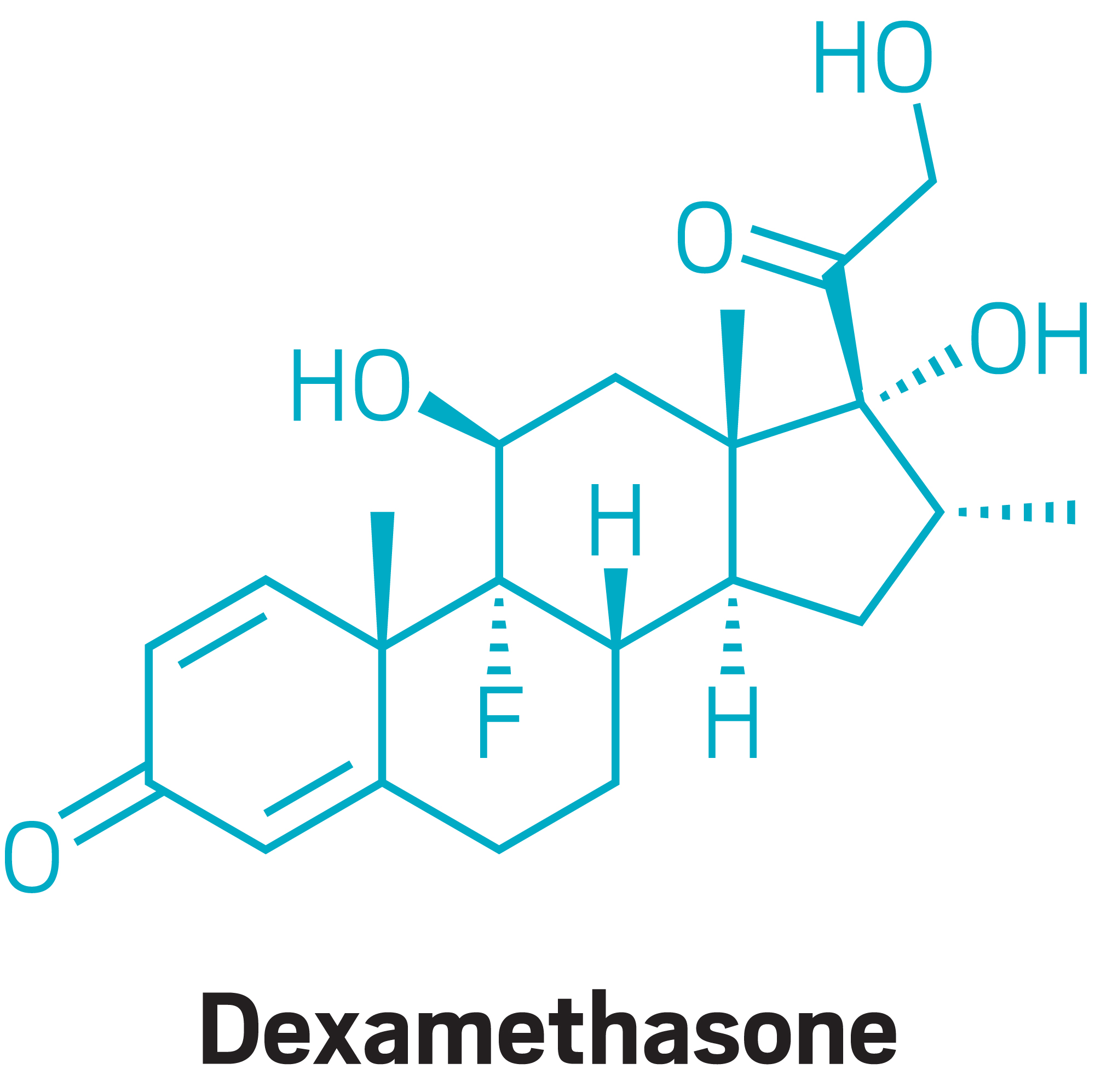
Early data from the UK RECOVERY trial suggest that the steroid dexamethasone can reduce deaths in severe cases of COVID-19. Read more in C&EN.
June 28, 2020
Pandemic milestones
Globally, 10 million people have been infected, and 500,000 people have died from COVID-19.
June 28, 2020
Vaccines
China approves CanSino's adenoviral vector vaccine for military use.
July 7, 2020
Pandemic milestones

Brazilian president Jair Bolsonaro tests positive for COVID-19.
August 11, 2020
Vaccines
Russia approves an unproven adenoviral vector vaccine manufactured by Binnopharm.
August 23, 2020
Drugs
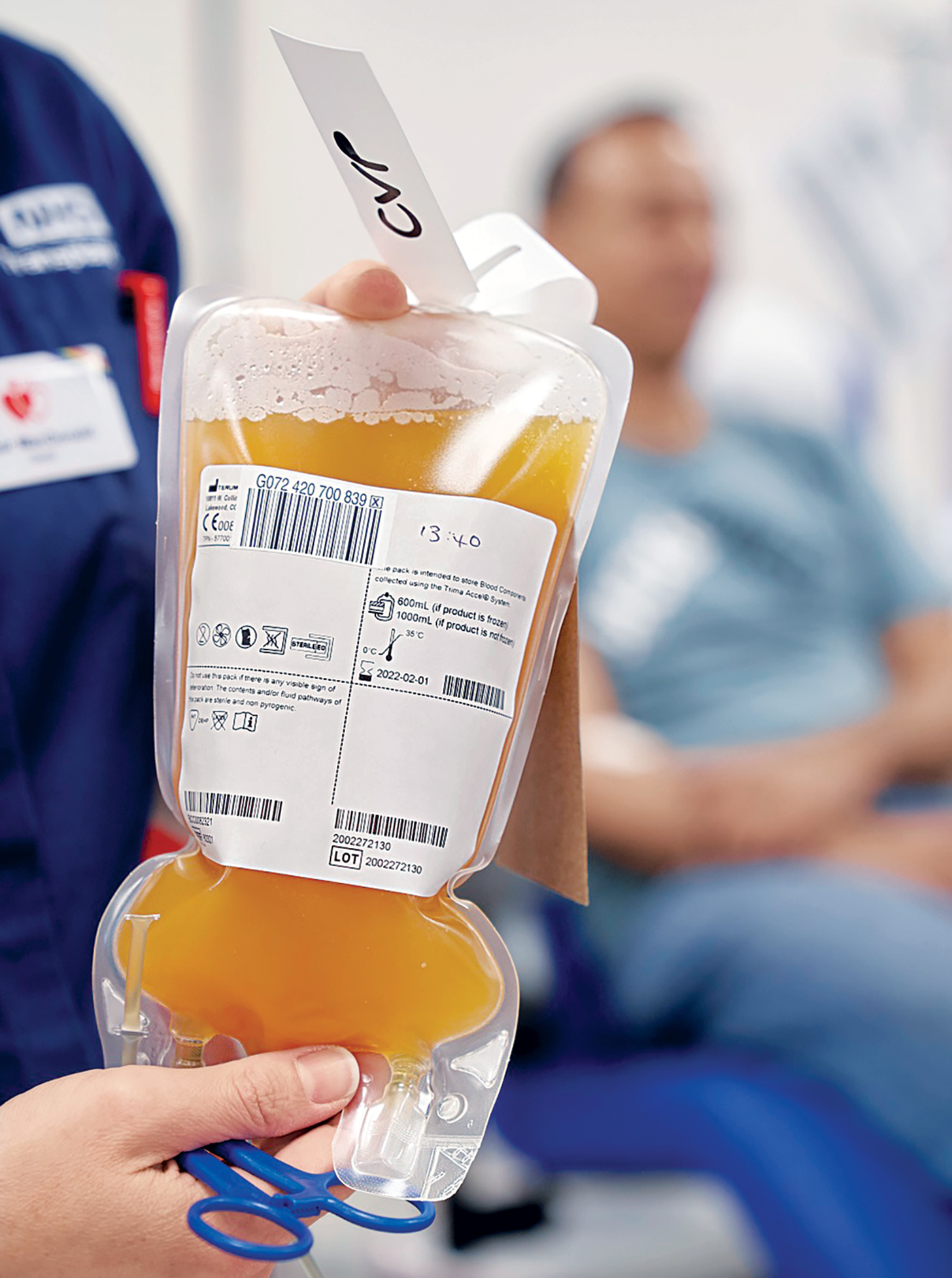
The FDA grants an EUA for convalescent plasma. Read more in C&EN.
September 3, 2020
Vaccines
A Phase 1/2 study of Sanofi and GlaxoSmithKline's recombinant subunit protein vaccine begins.
September 8, 2020
Vaccines
A Phase 3 study of AstraZeneca's adenoviral vector vaccine is put on hold after a participant experiences a serious adverse reaction.
September 22, 2020
Pandemic milestones
The US death toll crosses 200,000.
September 23, 2020
Vaccines
A Phase 3 study of Johnson & Johnson's adenoviral vector vaccine begins.
September 24, 2020
Vaccines
A Phase 3 study of Novavax's recombinant protein nanoparticle vaccine begins in the UK.
September 28, 2020
Pandemic milestones
Globally, more than 33 million people have been infected, and 1 million people have died from COVID-19.
October 1, 2020
Pandemic milestones

US president Donald J. Trump tests positive for COVID-19. Read more in C&EN.
October 12, 2020
Vaccines
Johnson & Johnson pauses a Phase 3 study of its adenoviral vector vaccine to investigate an unexplained illness in a participant.
October 22, 2020
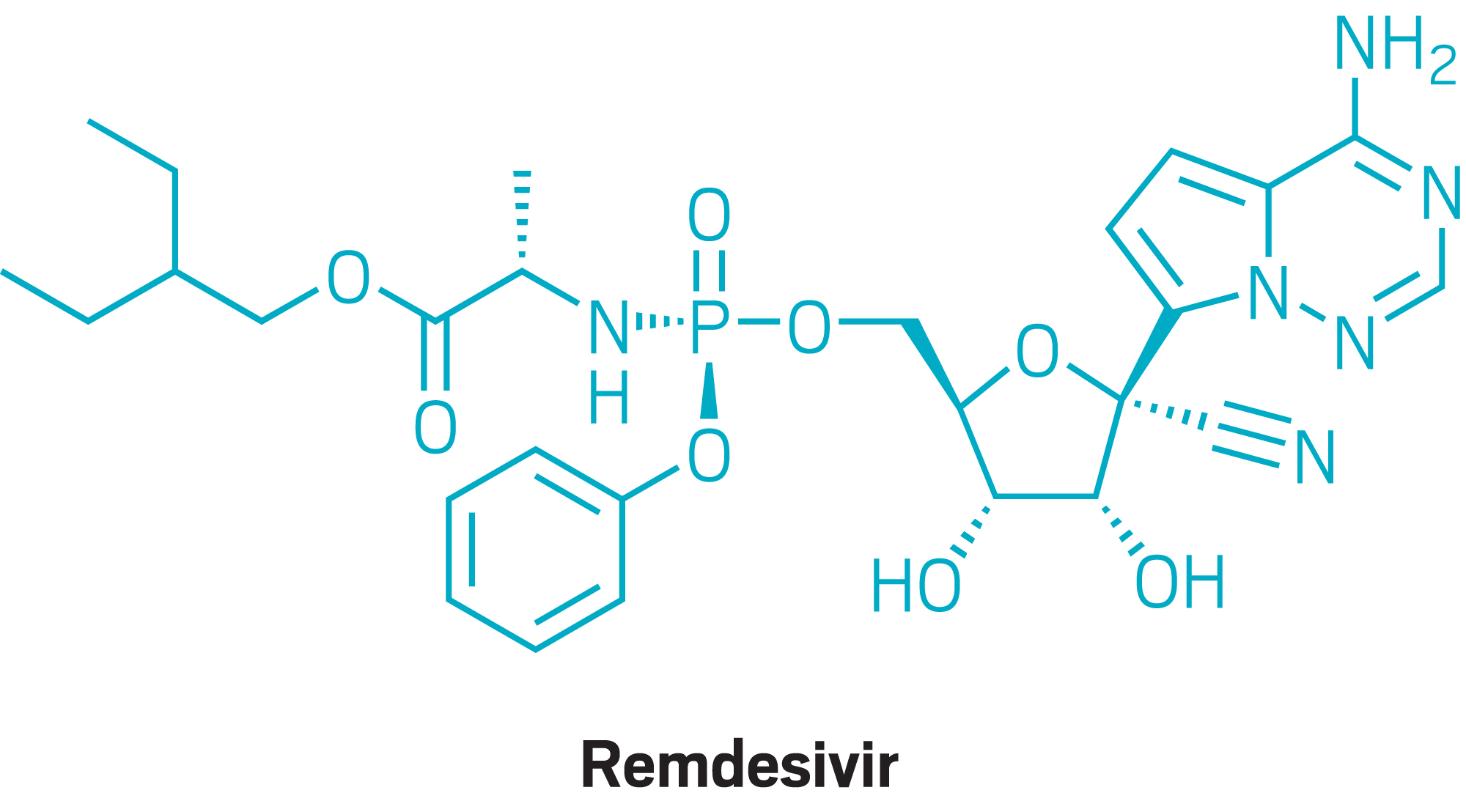
Drugs

Remdesivir becomes the first treatment to receive full FDA approval for COVID-19.
October 28, 2020
Drugs
Gilead Sciences reports that third-quarter sales of remdesivir were $873 million.
November 6, 2020
Diagnostics
The FDA grants an EUA for the first test that measures neutralizing antibodies from a recent or prior infection.
November 8, 2020
Pandemic milestones
Globally, more than 50 million people have been infected with COVID-19, and 1.25 million people have died from it.
November 8, 2020
Pandemic milestones

Newly declared US president-elect Joe Biden releases a seven-point COVID-19 plan. Read more in C&EN.
November 9, 2020
Vaccines
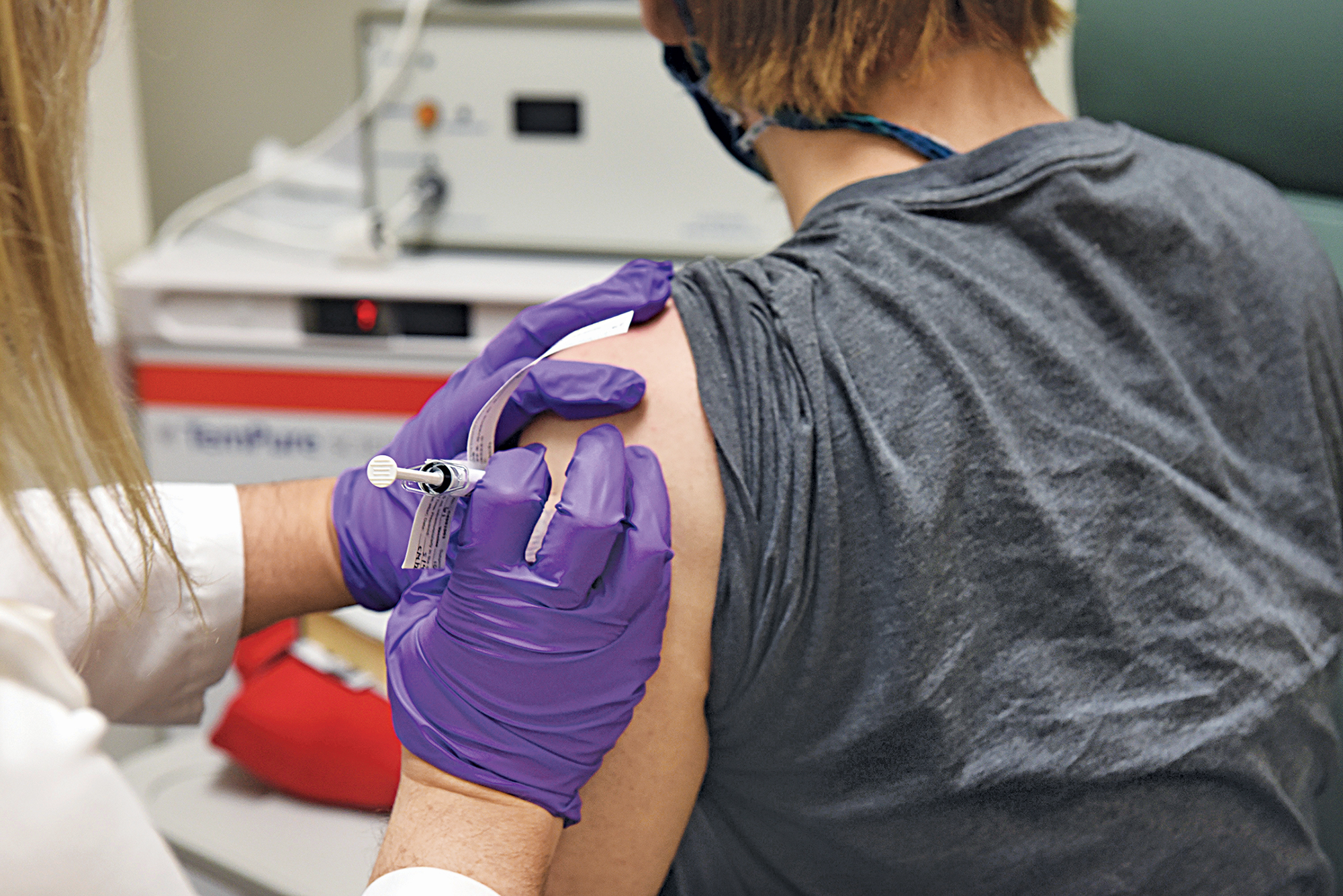
Pfizer says initial data from a Phase 3 study show its COVID-19 vaccine is 90% effective—a figure that is later increased to 95%. Read more in C&EN.
November 9, 2020
Drugs
The FDA grants an EUA for Lilly's monoclonal antibody treatment for recently diagnosed, high-risk COVID-19 patients. Read more in C&EN.
November 16, 2020
Vaccines

Moderna says initial data from a Phase 3 study show its mRNA vaccine is 94.5% effective. Read more in C&EN.
November 17, 2020
Diagnostics
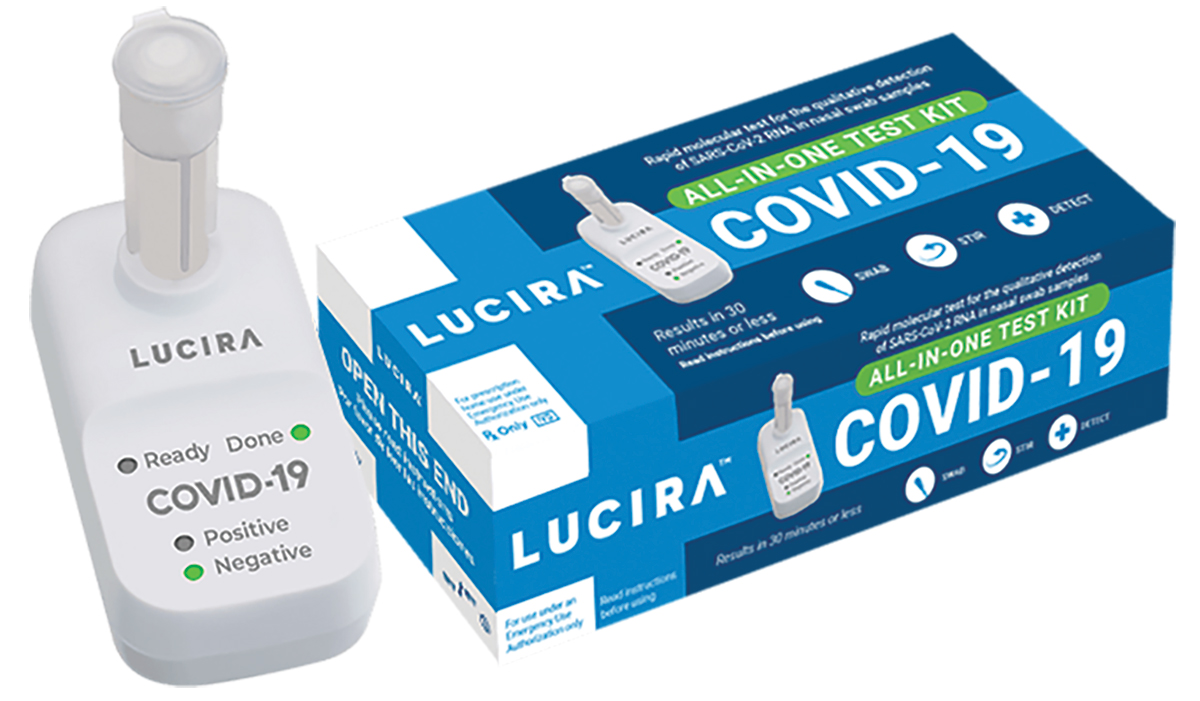
The FDA grants an EUA for the first self-administered diagnostic test that delivers results at home. Read more in C&EN.
November 19, 2020
Drugs
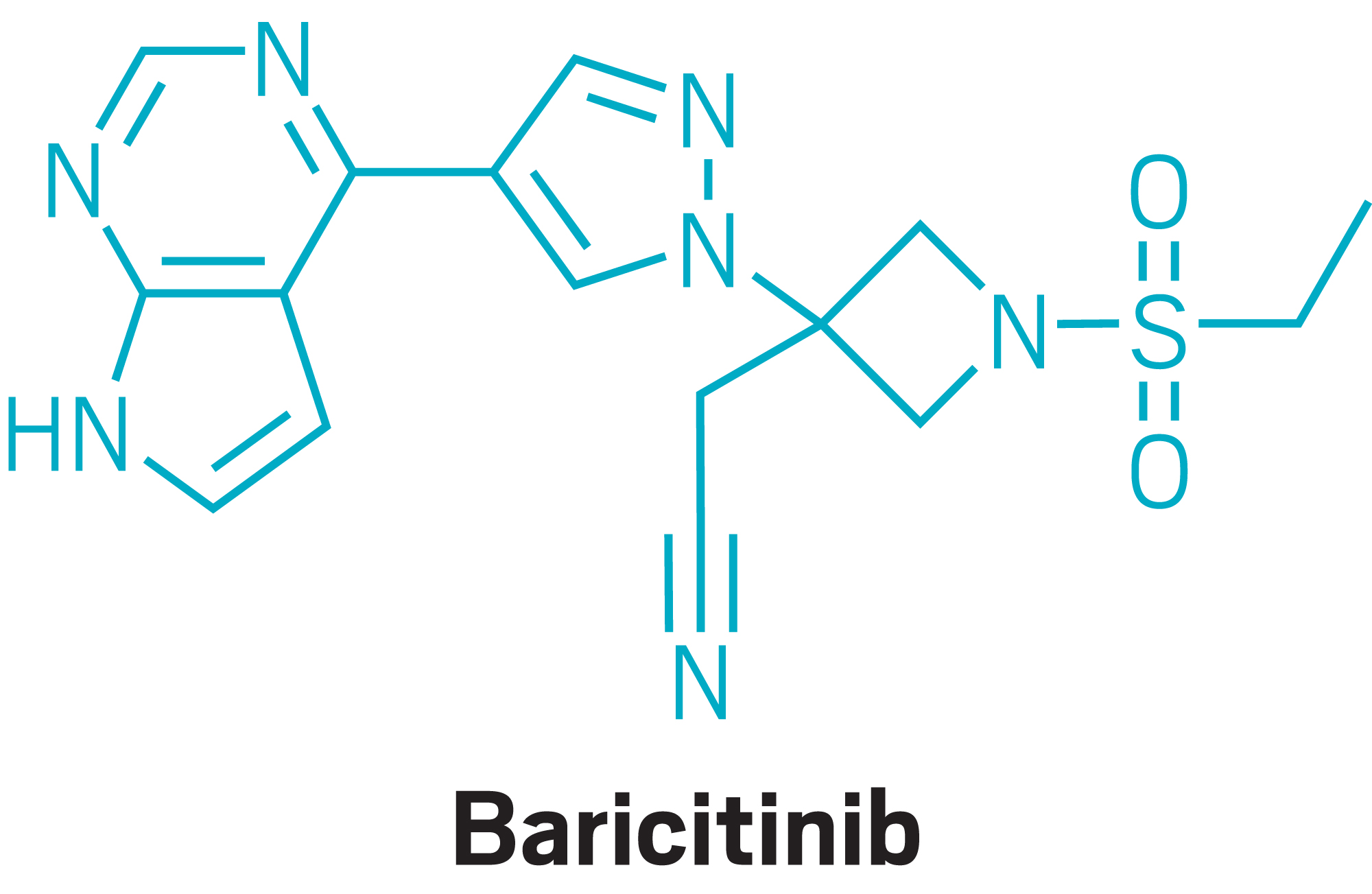
The FDA grants an EUA for Lilly's JAK inhibitor baricitinib, in combination with remdesivir, to treat people hospitalized with COVID-19.
November 20, 2020
Pandemic milestones
The US death toll crosses 250,000.
November 21, 2020
Drugs
The FDA grants an EUA for Regeneron's antibody therapy for use in high-risk people recently diagnosed with COVID-19.
November 22, 2020
Vaccines

AstraZeneca and the University of Oxford report positive but confusing initial Phase 3 data for their adenoviral vector vaccine. Read more in C&EN.
December 2, 2020
Vaccines
The UK grants emergency authorization for Pfizer and BioNTech's mRNA vaccine.
December 4, 2020
Diagnostics
The FDA grants an EUA for Quest Diagnostics' combination flu and COVID-19 test, the first combination kit to allow samples to be taken at home.
December 8, 2020
Vaccines
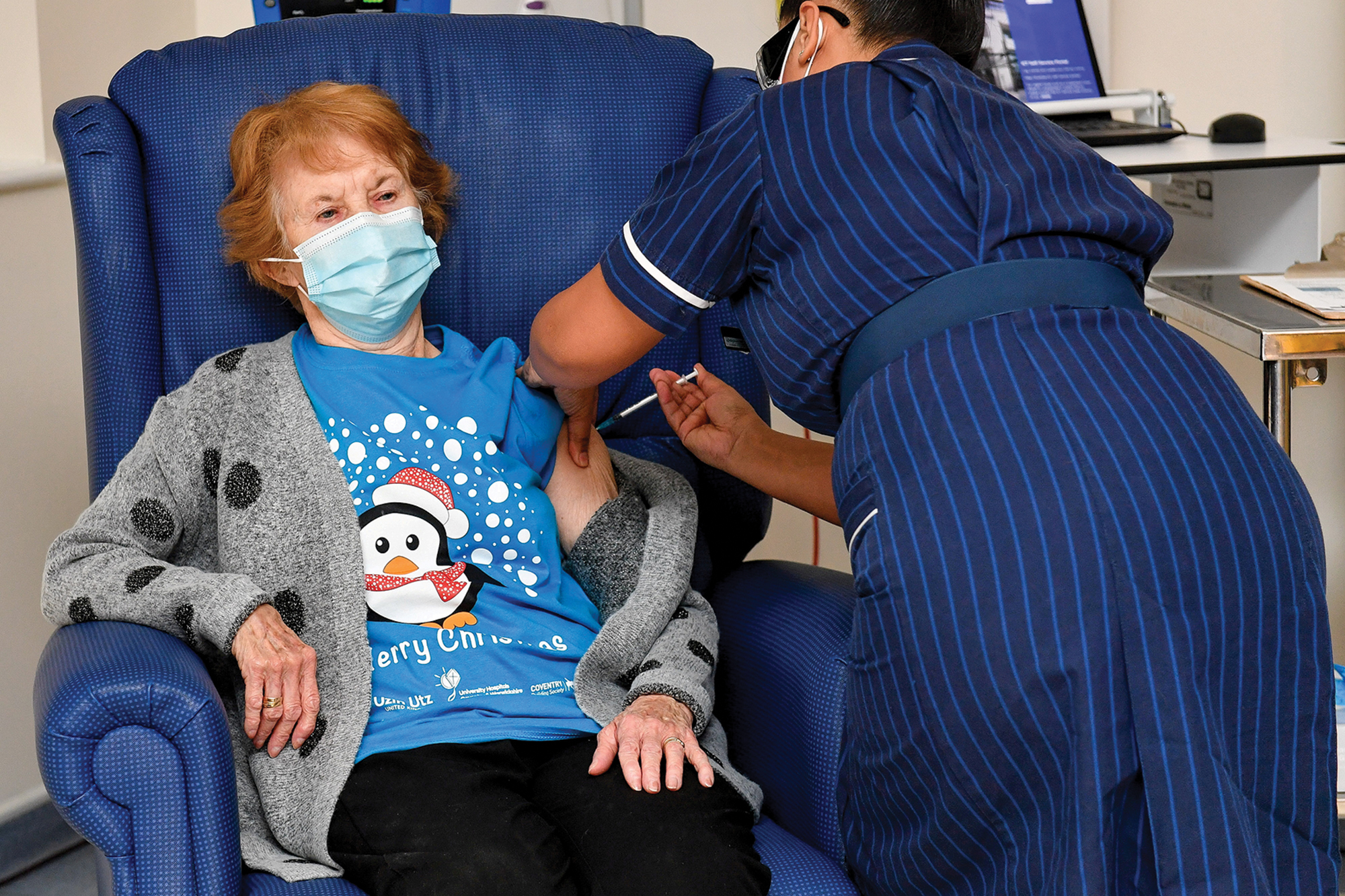
The rollout of Pfizer and BioNTech's mRNA vaccine begins in the UK.
December 9, 2020
Vaccines
Health Canada authorizes emergency use of Pfizer and BioNTech's mRNA vaccine.
December 10, 2020
Vaccines
Australia discontinues development of the University of Queensland's vaccine after it causes false positives on HIV tests.
December 11, 2020
Vaccines
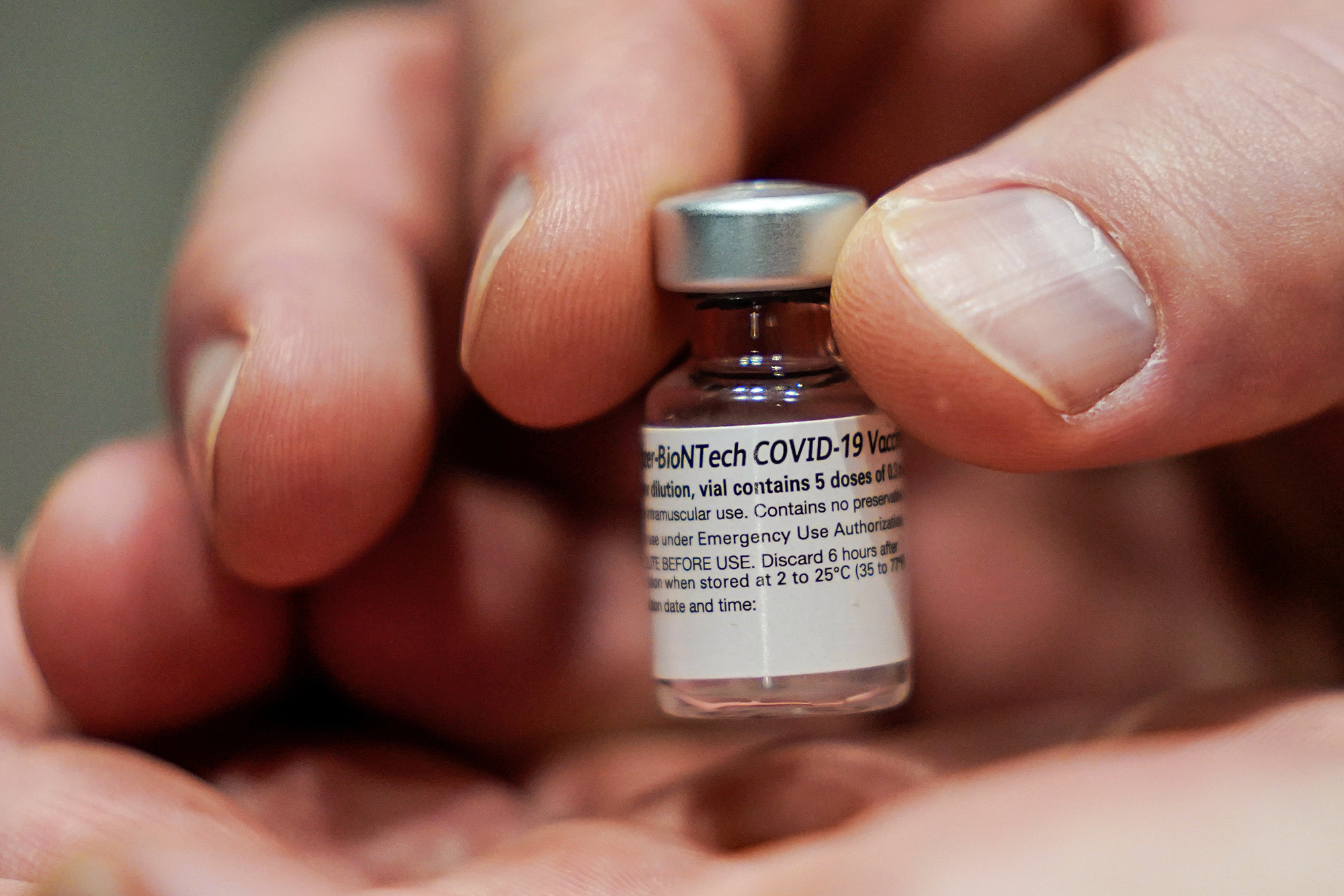
The FDA grants an EUA for Pfizer's mRNA vaccine, making it the first authorized COVID-19 vaccine in the US.
December 11, 2020
Vaccines
Poor efficacy in older adults prompts Sanofi and GSK to delay studies of their recombinant protein vaccine.
December 14, 2020
Pandemic milestones
The US death toll crosses 300,000.
December 14, 2020
Vaccines
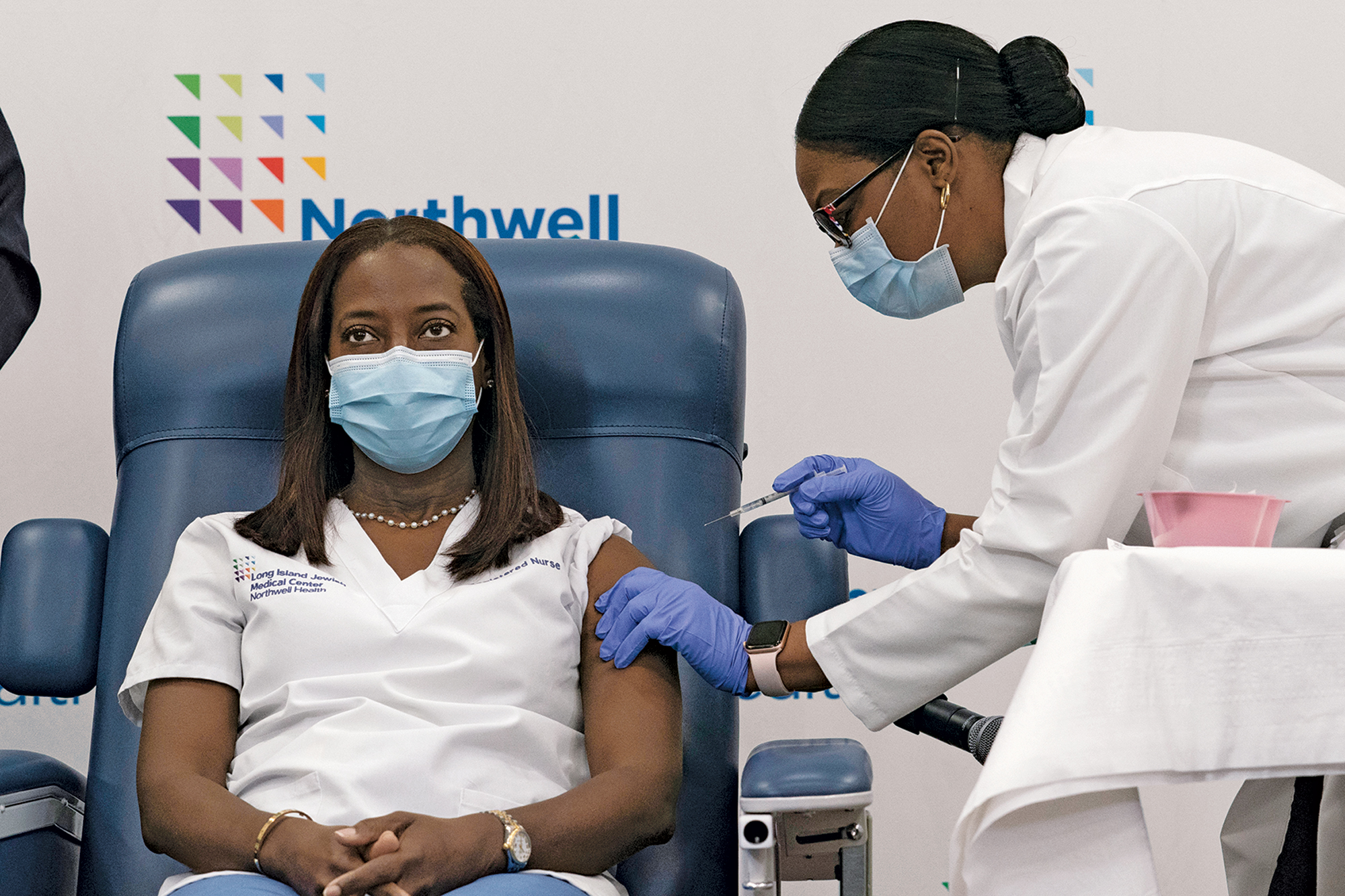
The rollout of Pfizer's vaccine begins in the US, where health workers are first to receive doses. Read more in C&EN.
December 15, 2020
Diagnostics
The FDA authorizes the first fully over-the-counter at-home diagnostic.
December 17, 2020
Pandemic milestones

French president Emmanuel Macron tests positive for COVID-19.
December 18, 2020
Vaccines
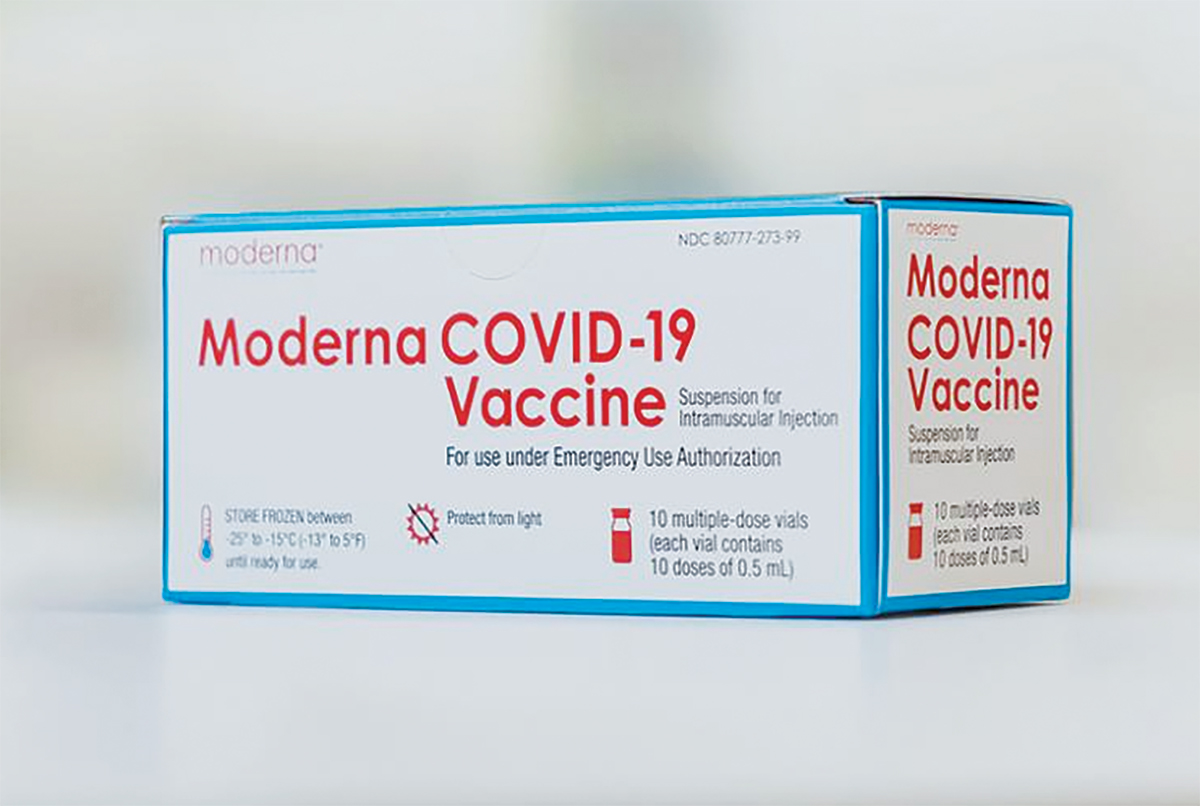
The FDA grants an EUA for Moderna's mRNA vaccine. Read more in C&EN.
December 21, 2020
Vaccines
The European Union authorizes Pfizer and BioNTech's mRNA vaccine.
December 28, 2020
Vaccines
A Phase 3 study of Novavax's recombinant protein vaccine begins in the US and Mexico.
December 30, 2020
Vaccines
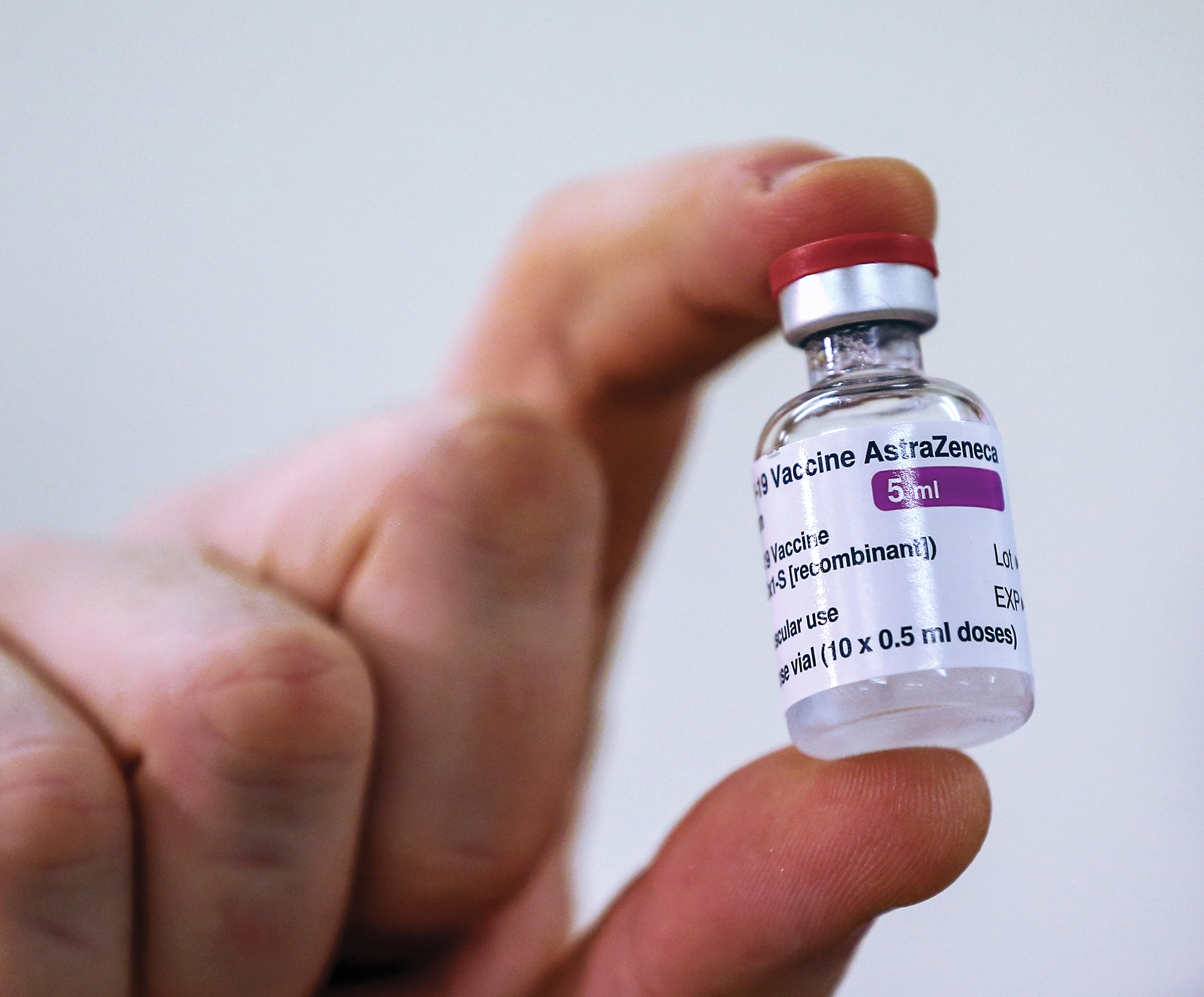
UK health authorities grant emergency authorization for AstraZeneca and the University of Oxford's adenoviral vector vaccine.
December 31, 2020
Vaccines

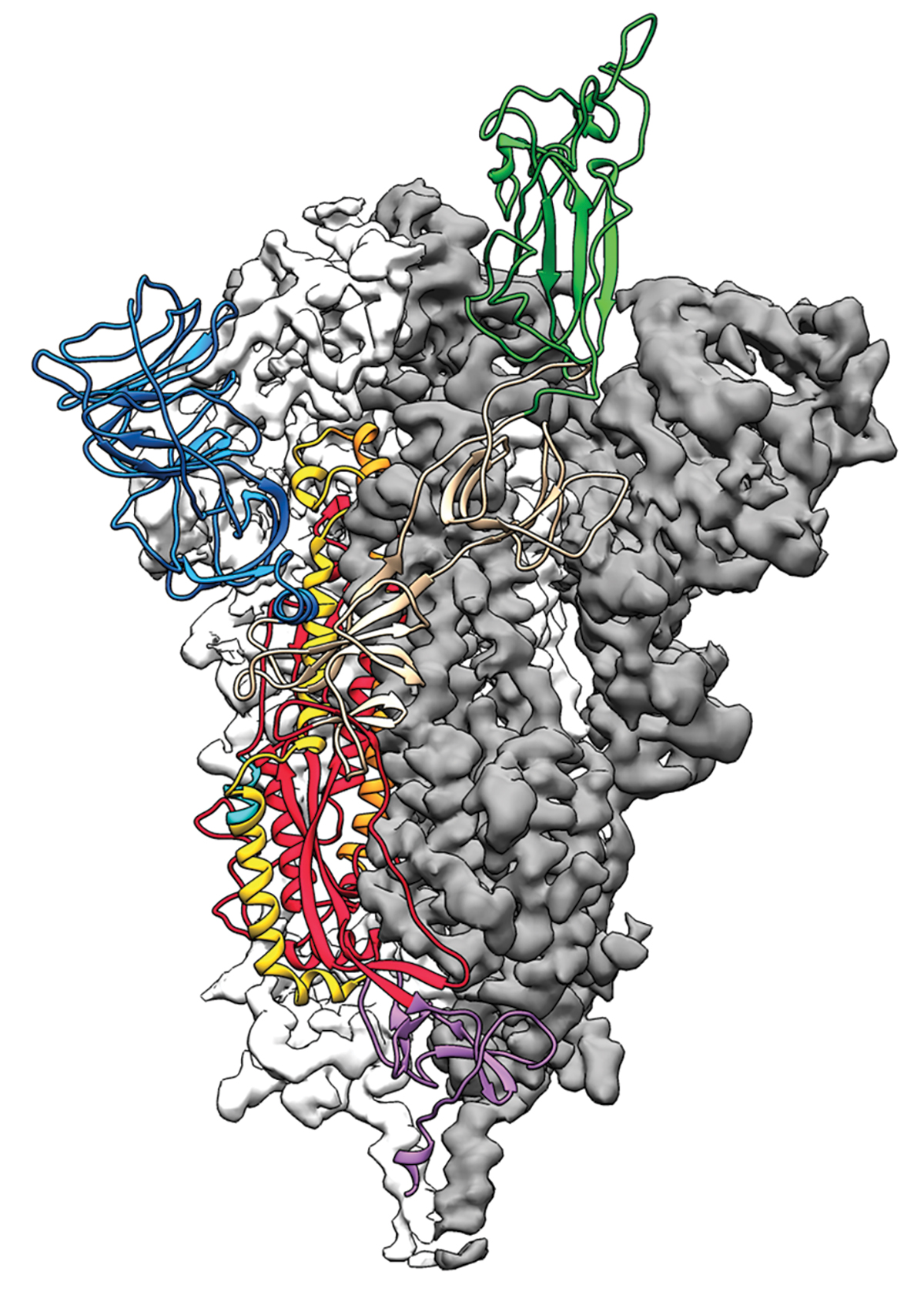
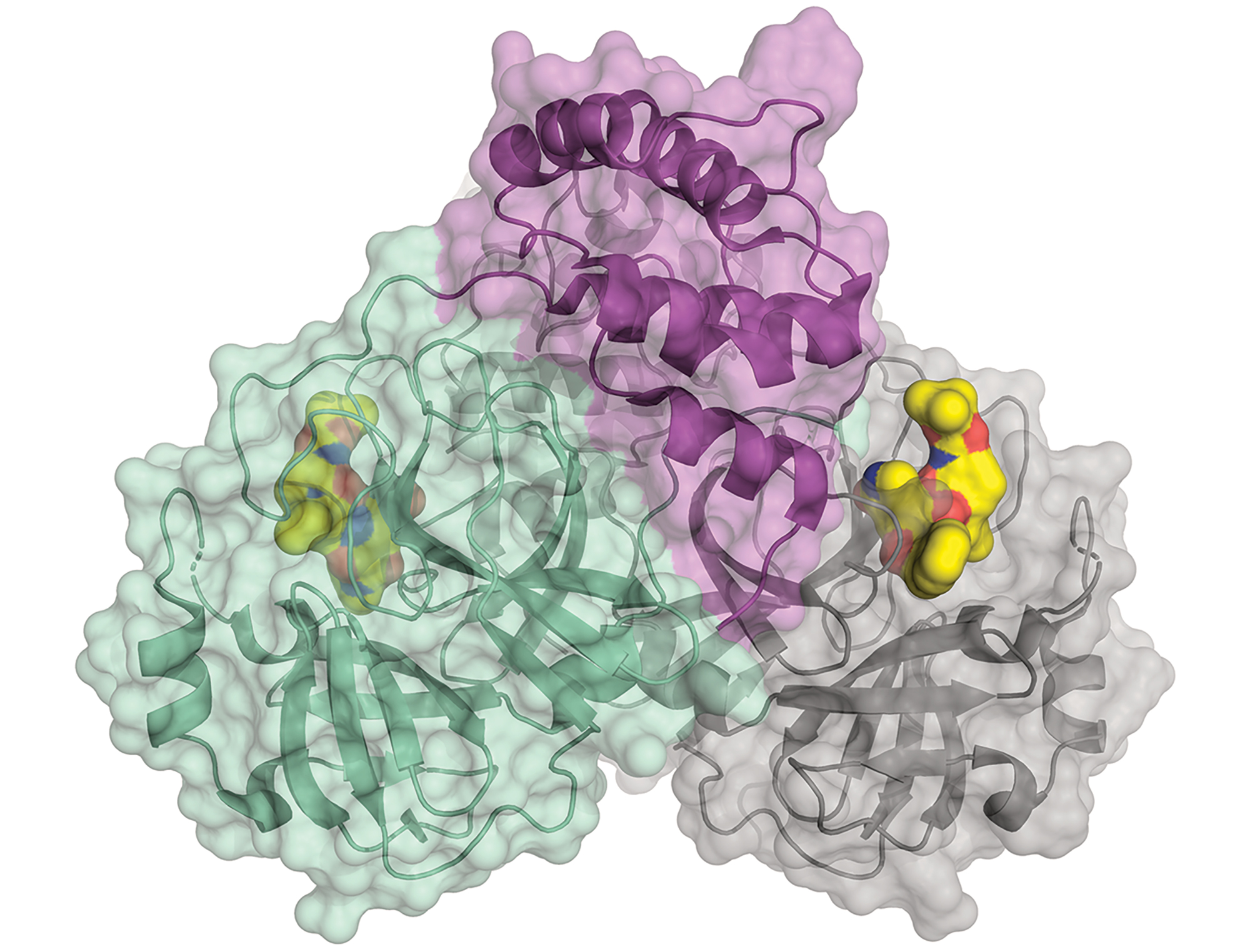
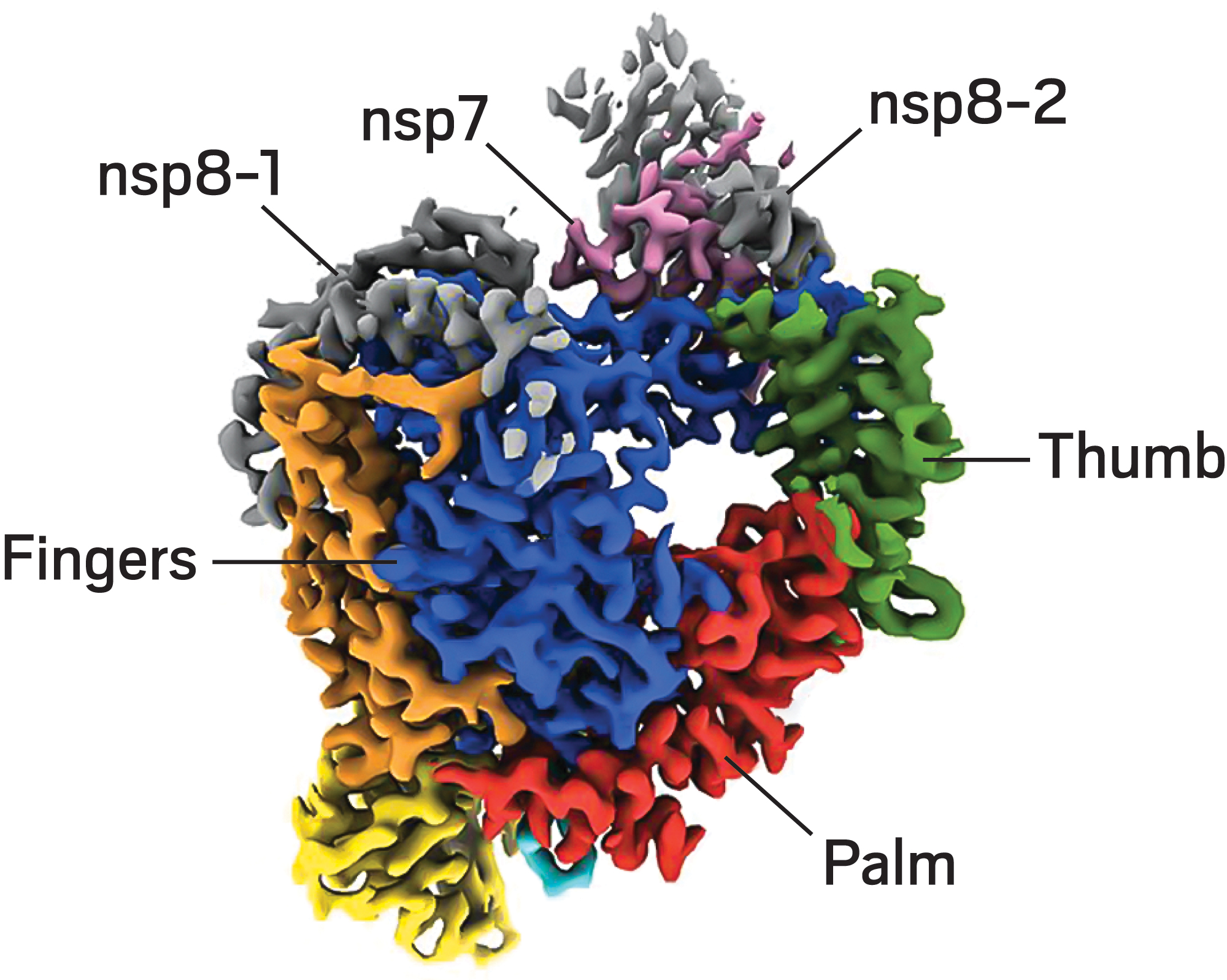



Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter