Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
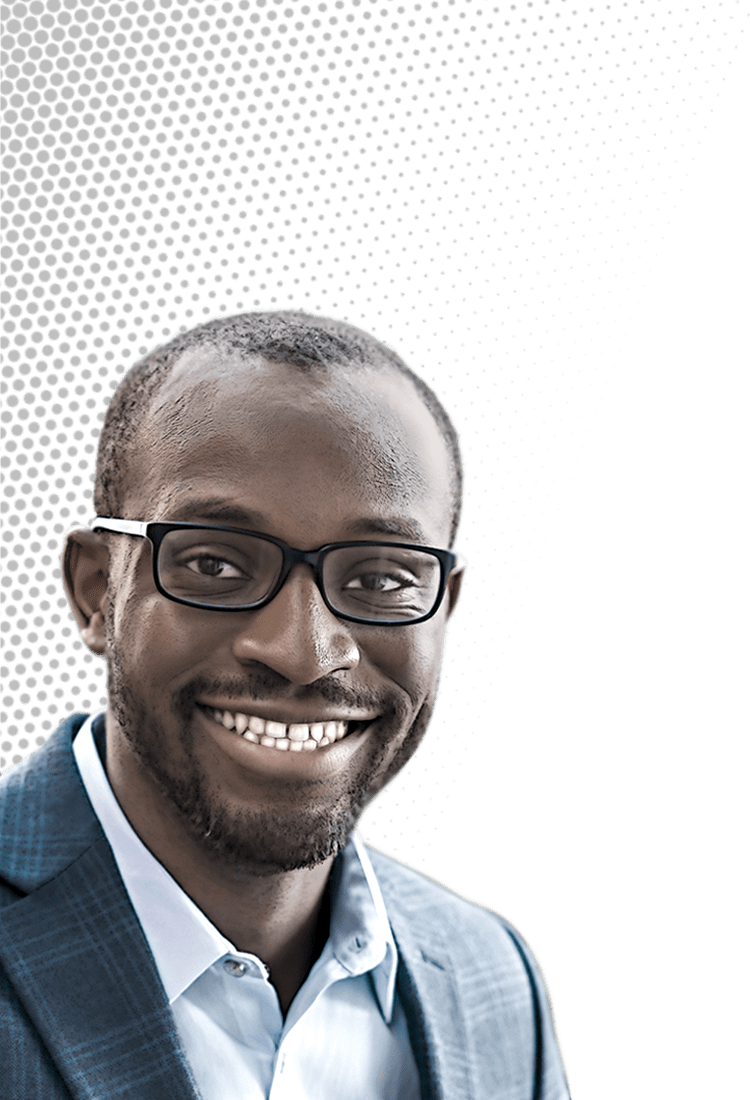
Chibueze Amanchukwu likes to focus on a somewhat neglected realm of battery research. To produce more-efficient and longer-lasting batteries to store the electricity generated by the renewable energy boom, researchers have delivered a slew of improvements in electrode materials and other key components. But electrolytes, which carry ions back and forth between electrodes, have often been overlooked.
Vitals
Current affiliation: University of Chicago
Age: 31
PhD alma mater: Massachusetts Institute of Technology
Hometown: Lagos, Nigeria
If I were an element, I’d be: “Can it be anything other than lithium? An element that has given me so much! It was my first entrance into the world of batteries and set me on the path I have been on for over a decade.”
My favorite TV show is: “The Wire. I find it heart wrenching, as I have shed many a tear watching the show multiple times. It features so many unforgettable characters and themes that parallel ‘conventional’ society and shows how society can both build up and tear down dreams.”
Amanchukwu aims to change that. His team at the University of Chicago is developing entirely new classes of electrolytes to boost the efficiency of conventional lithium-ion batteries as well as next-generation lithium-metal batteries. He’s also developing electrolytes for other forms of energy storage, including electrochemical cells that convert carbon dioxide into useful products. “He’s a wonderful, enthusiastic individual and an exceptionally talented scientist,” says Zhenan Bao of Stanford University, Amanchukwu’s postdoctoral supervisor.
Born in Lagos, Nigeria, Amanchukwu moved with his family to Houston when he was a teenager. He was enrolled in classes there that were commensurate with his talent rather than his age. As a result, he graduated from high school at 15 and completed his undergraduate degree in chemical engineering at Texas A&M University at just 19. After catching the battery bug during an internship at DuPont, he focused on lithium-air batteries while doing his PhD work with Paula Hammond at the Massachusetts Institute of Technology.
Conventional lithium-ion batteries typically use an electrolyte of lithium salts in organic solvents such as ethylene carbonate to shuttle lithium ions between a graphite anode and a metal oxide cathode. Lithium-metal batteries are lighter because they contain lithium-metal anodes instead of graphite. Lithium-air batteries go one step further in shaving off weight by using a porous carbon cathode to host reactions between oxygen and lithium ions during charging. In principle, a lithium-air battery could store roughly 10 times as much energy as a lithium-ion battery of the same weight.
The downside is that lithium and oxygen combine to produce superoxide and peroxide species that are highly reactive and degrade both the cathode material and the electrolyte. “Controlling that reactivity has been the main challenge that continues to plague lithium-air batteries,” Amanchukwu says.
During his postdoc with Bao, Amanchukwu developed fluorinated ether solvents that are stable against these superoxides and peroxides and that enable lithium to enter and leave the metal anode more efficiently. Since establishing his own research group in 2020, he has continued to fine-tune the fluoroethers to further enhance their properties and has found that they also enable faster charging and a wider range of operating temperatures in good old lithium-ion batteries.
“The degradation chemistry of electrolytes has not been considered to be all that exciting,” says the University of Cambridge battery scientist Clare Grey, who hosted Amanchukwu as a visiting fellow in 2019. “But he’s part of a cohort bringing in new synthesis and characterization methods, and just some good physical chemistry, into the space.”
Advertisement
Still, some electrolyte solvents have downsides like volatility and flammability. So Amanchukwu has developed a series of solvent-free electrolytes that are based on nonvolatile and nonflammable salts that melt at relatively low temperatures. Lithium-metal batteries equipped with these molten salt electrolytes can operate at 80 °C, and Amanchukwu says recent unpublished improvements from his lab have reduced that to ambient temperature.
He’s also using electrolyte chemistry to improve electrochemical systems that convert CO2 into useful compounds. These systems take electricity generated from solar or wind farms and then use water as a source of protons to form products such as ethylene or ethanol. Unfortunately, these electrochemical cells also generate a lot of hydrogen gas, which lowers process efficiency. His team has tried to increase efficiency by adding simple salts and organic solvents to the electrolyte—a tweak that suppresses hydrogen formation while boosting CO2 conversion.
In addition to pursuing his electrolyte revolution, Amanchukwu is nurturing the next generation of chemists who might work on sustainable energy technologies. He founded Research Experience for Nigerian Engineering Undergraduates, a program that matches mentors in the US with talented students to give them research experience and help them be more competitive when applying for PhD programs. “The Industrial Revolution really left certain parts of the world behind,” he says. “We cannot allow the renewable energy revolution that is happening now to leave us further behind again.”
Energy Storage
Chibueze Amanchukwu
This electrochemist invents new electrolytes to boost batteries
by Mark Peplow, special to C&EN
May 17, 2024
| A version of this story appeared in
Volume 102, Issue 15

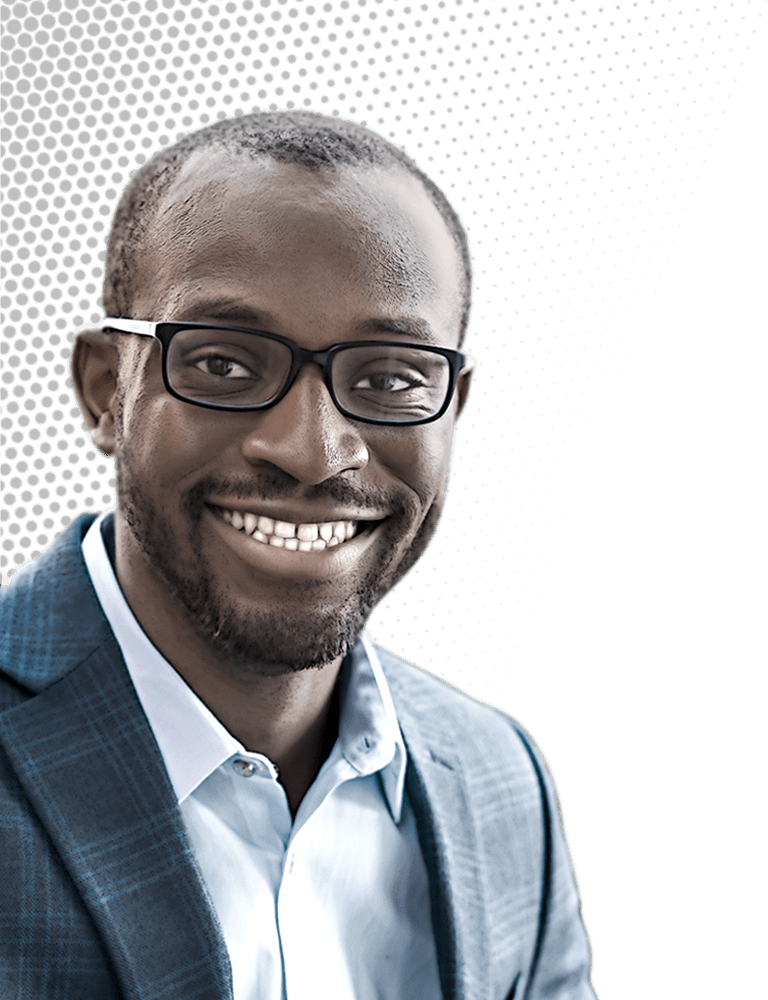
Credit: Daniel Ajadi/C&EN | Chibueze Amanchukwu
Vitals
Current affiliation: University of Chicago
Age: 31
PhD alma mater: Massachusetts Institute of Technology
Hometown: Lagos, Nigeria
If I were an element, I’d be: “Can it be anything other than lithium? An element that has given me so much! It was my first entrance into the world of batteries and set me on the path I have been on for over a decade.”
My favorite TV show is: “The Wire. I find it heart wrenching, as I have shed many a tear watching the show multiple times. It features so many unforgettable characters and themes that parallel ‘conventional’ society and shows how society can both build up and tear down dreams.”
Chibueze Amanchukwu likes to focus on a somewhat neglected realm of battery research. To produce more-efficient and longer-lasting batteries to store the electricity generated by the renewable energy boom, researchers have delivered a slew of improvements in electrode materials and other key components. But electrolytes, which carry ions back and forth between electrodes, have often been overlooked.
Amanchukwu aims to change that. His team at the University of Chicago is developing entirely new classes of electrolytes to boost the efficiency of conventional lithium-ion batteries as well as next-generation lithium-metal batteries. He’s also developing electrolytes for other forms of energy storage, including electrochemical cells that convert carbon dioxide into useful products. “He’s a wonderful, enthusiastic individual and an exceptionally talented scientist,” says Zhenan Bao of Stanford University, Amanchukwu’s postdoctoral supervisor.
Born in Lagos, Nigeria, Amanchukwu moved with his family to Houston when he was a teenager. He was enrolled in classes there that were commensurate with his talent rather than his age. As a result, he graduated from high school at 15 and completed his undergraduate degree in chemical engineering at Texas A&M University at just 19. After catching the battery bug during an internship at DuPont, he focused on lithium-air batteries while doing his PhD work with Paula Hammond at the Massachusetts Institute of Technology.
Conventional lithium-ion batteries typically use an electrolyte of lithium salts in organic solvents such as ethylene carbonate to shuttle lithium ions between a graphite anode and a metal oxide cathode. Lithium-metal batteries are lighter because they contain lithium-metal anodes instead of graphite. Lithium-air batteries go one step further in shaving off weight by using a porous carbon cathode to host reactions between oxygen and lithium ions during charging. In principle, a lithium-air battery could store roughly 10 times as much energy as a lithium-ion battery of the same weight.
The downside is that lithium and oxygen combine to produce superoxide and peroxide species that are highly reactive and degrade both the cathode material and the electrolyte. “Controlling that reactivity has been the main challenge that continues to plague lithium-air batteries,” Amanchukwu says.
During his postdoc with Bao, Amanchukwu developed fluorinated ether solvents that are stable against these superoxides and peroxides and that enable lithium to enter and leave the metal anode more efficiently. Since establishing his own research group in 2020, he has continued to fine-tune the fluoroethers to further enhance their properties and has found that they also enable faster charging and a wider range of operating temperatures in good old lithium-ion batteries.
“The degradation chemistry of electrolytes has not been considered to be all that exciting,” says the University of Cambridge battery scientist Clare Grey, who hosted Amanchukwu as a visiting fellow in 2019. “But he’s part of a cohort bringing in new synthesis and characterization methods, and just some good physical chemistry, into the space.”
Still, some electrolyte solvents have downsides like volatility and flammability. So Amanchukwu has developed a series of solvent-free electrolytes that are based on nonvolatile and nonflammable salts that melt at relatively low temperatures. Lithium-metal batteries equipped with these molten salt electrolytes can operate at 80 °C, and Amanchukwu says recent unpublished improvements from his lab have reduced that to ambient temperature.
He’s also using electrolyte chemistry to improve electrochemical systems that convert CO2into useful compounds. These systems take electricity generated from solar or wind farms and then use water as a source of protons to form products such as ethylene or ethanol. Unfortunately, these electrochemical cells also generate a lot of hydrogen gas, which lowers process efficiency. His team has tried to increase efficiency by adding simple salts and organic solvents to the electrolyte—a tweak that suppresses hydrogen formation while boosting CO2conversion.
In addition to pursuing his electrolyte revolution, Amanchukwu is nurturing the next generation of chemists who might work on sustainable energy technologies. He founded Research Experience for Nigerian Engineering Undergraduates, a program that matches mentors in the US with talented students to give them research experience and help them be more competitive when applying for PhD programs. “The Industrial Revolution really left certain parts of the world behind,” he says. “We cannot allow the renewable energy revolution that is happening now to leave us further behind again.”






Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter