Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Materials
Lauren Zarzar
Materials magician designs liquid droplets that could act as microscopic lenses and iridescent coatings
by Michael Torrice
August 25, 2019
| A version of this story appeared in
Volume 97, Issue 33


Vitals
Current affiliation: Pennsylvania State University
Age: 33
PhD alma mater: Harvard University
Role model: “Sylvia Earle, a marine biologist and explorer. I read her books and attended one of her lectures when I was young. While I didn’t end up going into marine biology, she inspired me to try to be an explorer and pioneer in my own field.”
If I were an element, I would be: “My favorite element is fluorine. Replacing hydrogen in organic compounds with fluorine gives molecules really unusual chemical and material properties. But if I were an element, I’d be bismuth, because the surface oxidizes and it just looks really awesome.”
Must-have in the lab: “Microscopes—our eyes to another world!”
Must-have on the road: “Mobile hot spot.”
3 key papers
“Colouration by Total Internal Reflection and Interference at Microscale Concave Interfaces” (Nature2019, DOI: 10.1038/s41586-019-0946-4)
“Using Laser-Induced Thermal Voxels to Pattern Diverse Materials at the Solid-Liquid Interface” (ACS Appl. Mater. Interfaces2016, DOI: 10.1021/acsami.6b06625)
“Dynamically Reconfigurable Complex Emulsions via Tunable Interfacial Tensions” (Nature2015, DOI: 10.1038/nature14168)
Lauren Zarzar thinks liquids deserve some materials attention.
“Liquids are the most dynamic and responsive materials we have,” the Pennsylvania State University researcher says. Unlike solids, she explains, liquids change shape easily, and two liquids in contact can essentially communicate, passing molecules between each other.
Zarzar has developed ways to control the shapes and compositions of tiny droplets of complex emulsions. She thinks these liquid materials could act as microscopic lenses, simple sensors, and colorful coatings.
The droplets are also part of a research theme that Zarzar has pursued since she was a graduate student at Harvard University: materials that can respond to their surroundings. Most materials, Zarzar says, are static and designed for a specific purpose. But materials aren’t always used under one set of circumstances, she says. “If they could adapt and change, we could make materials that are more functional and useful.”
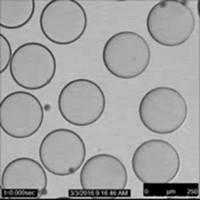
As a postdoc at the Massachusetts Institute of Technology, Zarzar started working with complex emulsions. These droplets are made of liquids that don’t mix, such as a droplet containing a fluorocarbon oil inside a hydrocarbon oil floating in water.
She developed an easy way to build these droplets and then change their structure. For example, she could pick which oil was on the inside of the droplet and which was on the outside. She started with oils that didn’t mix at room temperature but could when heated. By cooling these heated mixtures with surfactants, she could control the structure of the emulsions as the oils stopped mixing. By adding different surfactants to a solution containing the emulsions, she could change the droplets’ structure again—flipping which oil was inside the droplet and which was outside, for example.
Surfactants influence the droplets’ structure by controlling the surface tension of the different liquids in the emulsions. That surface tension also dictates the curvature of the droplets, which can influence how light interacts with the materials, affecting their optical properties. Researchers could use the droplets as lenses and tune them as they would a microscope.
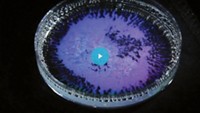
Zarzar also recently reported a colorful example of the droplets’ optical properties.
Light bounces off the curved surfaces of these droplets and causes interference, leading to iridescence, as you might see in soap bubbles. Zarzar and her lab found that they could control the curvature of their droplets by adding different surfactants, allowing them to change this iridescence. They describe this effect as a tunable version of structural color, which is color that arises not from dyes but from how light interacts with the structure of a material, like the color on some butterfly wings.
“You can tune that curvature in the droplets dynamically, and that changes the color, which you can’t do easily in a solid,” Zarzar says. She thinks this phenomenon could be exploited in colorful coatings and reflective displays.
The work, published earlier this year, “showed Lauren’s creativity and ability to bring together orthogonal fields, thinking how chemistry can be combined with materials science and with optics to come up with ideas that don’t fit in just one field,” says Joanna Aizenberg, who was Zarzar’s graduate adviser at Harvard.
Zarzar plans to keep pursuing the theme of responsive materials and think about ways to create what she calls artificial living materials—materials that can adapt and evolve over time.
Lauren Zarzar thinks liquids deserve some materials attention.
“Liquids are the most dynamic and responsive materials we have,” the Pennsylvania State University researcher says. Unlike solids, she explains, liquids change shape easily, and two liquids in contact can essentially communicate, passing molecules between each other.
Advertisement
Zarzar has developed ways to control the shapes and compositions of tiny droplets of complex emulsions. She thinks these liquid materials could act as microscopic lenses, simple sensors, and colorful coatings.
The droplets are also part of a research theme that Zarzar has pursued since she was a graduate student at Harvard University: materials that can respond to their surroundings. Most materials, Zarzar says, are static and designed for a specific purpose. But materials aren’t always used under one set of circumstances, she says. “If they could adapt and change, we could make materials that are more functional and useful.”
As a postdoc at the Massachusetts Institute of Technology, Zarzar started working with complex emulsions. These droplets are made of liquids that don’t mix, such as a droplet containing a fluorocarbon oil inside a hydrocarbon oil floating in water.
She developed an easy way to build these droplets and then change their structure. For example, she could pick which oil was on the inside of the droplet and which was on the outside. She started with oils that didn’t mix at room temperature but could when heated. By cooling these heated mixtures with surfactants, she could control the structure of the emulsions as the oils stopped mixing. By adding different surfactants to a solution containing the emulsions, she could change the droplets’ structure again—flipping which oil was inside the droplet and which was outside, for example.
Surfactants influence the droplets’ structure by controlling the surface tension of the different liquids in the emulsions. That surface tension also dictates the curvature of the droplets, which can influence how light interacts with the materials, affecting their optical properties. Researchers could use the droplets as lenses and tune them as they would a microscope.
Zarzar also recently reported a colorful example of the droplets’ optical properties.
Light bounces off the curved surfaces of these droplets and causes interference, leading to iridescence, as you might see in soap bubbles. Zarzar and her lab found that they could control the curvature of their droplets by adding different surfactants, allowing them to change this iridescence. They describe this effect as a tunable version of structural color, which is color that arises not from dyes but from how light interacts with the structure of a material, like the color on some butterfly wings.
“You can tune that curvature in the droplets dynamically, and that changes the color, which you can’t do easily in a solid,” Zarzar says. She thinks this phenomenon could be exploited in colorful coatings and reflective displays.
The work, published earlier this year, “showed Lauren’s creativity and ability to bring together orthogonal fields, thinking how chemistry can be combined with materials science and with optics to come up with ideas that don’t fit in just one field,” says Joanna Aizenberg, who was Zarzar’s graduate adviser at Harvard.
Zarzar plans to keep pursuing the theme of responsive materials and think about ways to create what she calls artificial living materials—materials that can adapt and evolve over time.
Vitals
Current affiliation: Pennsylvania State University
Age: 33
PhD alma mater: Harvard University
Role model: “Sylvia Earle, a marine biologist and explorer. I read her books and attended one of her lectures when I was young. While I didn’t end up going into marine biology, she inspired me to try to be an explorer and pioneer in my own field.”
If I were an element, I would be: “My favorite element is fluorine. Replacing hydrogen in organic compounds with fluorine gives molecules really unusual chemical and material properties. But if I were an element, I’d be bismuth, because the surface oxidizes and it just looks really awesome.”
Must-have in the lab: “Microscopes—our eyes to another world!”
Must-have on the road: “Mobile hot spot.”
Research at a glance

Credit: Adapted from Nature/Yang H. Ku/C&EN
Zarzar makes emulsion droplets by heating two oils, forcing them to mix. By adding surfactants and letting the mixture cool, she can control the structure of the droplets, tuning their properties.


















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter