Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
Maher El-Kady
This materials scientist sculpts graphene with light to solve energy storage problems
by Mitch Jacoby
July 15, 2022
| A version of this story appeared in
Volume 100, Issue 25

Credit: Will Ludwig/C&EN/Tim Peacock/Maher El-Kady
Maher El-Kady is on a mission to reduce the world’s dependence on fossil fuels. To do so, he wants to build what he calls a “wonder battery” to power unprecedented numbers of electric vehicles.
“To speed up adoption of electric cars, we need batteries that hold enough charge to provide a long driving range, can be recharged quickly, and have a long life,” he says. “But they have to be safe—really safe—and affordable too.”
Advertisement
El-Kady thinks the key to making batteries with those specifications is a unique form of graphene and a simple way of synthesizing it. He discovered this process as a chemistry graduate student at the University of California, Los Angeles, where he now holds a position as a research professor. And today he’s making lithium-ion batteries with the material at Nanotech Energy, a start-up he cofounded and where he serves as chief technology officer.
“Maher is incredibly productive and an unbelievably good scientist—the best grad student I have ever had,” UCLA’s Richard B. Kaner says. Kaner notes that El-Kady has already coauthored nearly 200 patents and patent applications and published roughly 60 papers. He’s doing a top-notch job managing a team of 40 people, says Kaner, who serves as chair of Nanotech Energy’s scientific advisory board. “He’s mild mannered and the most creative guy you’ll ever meet.”
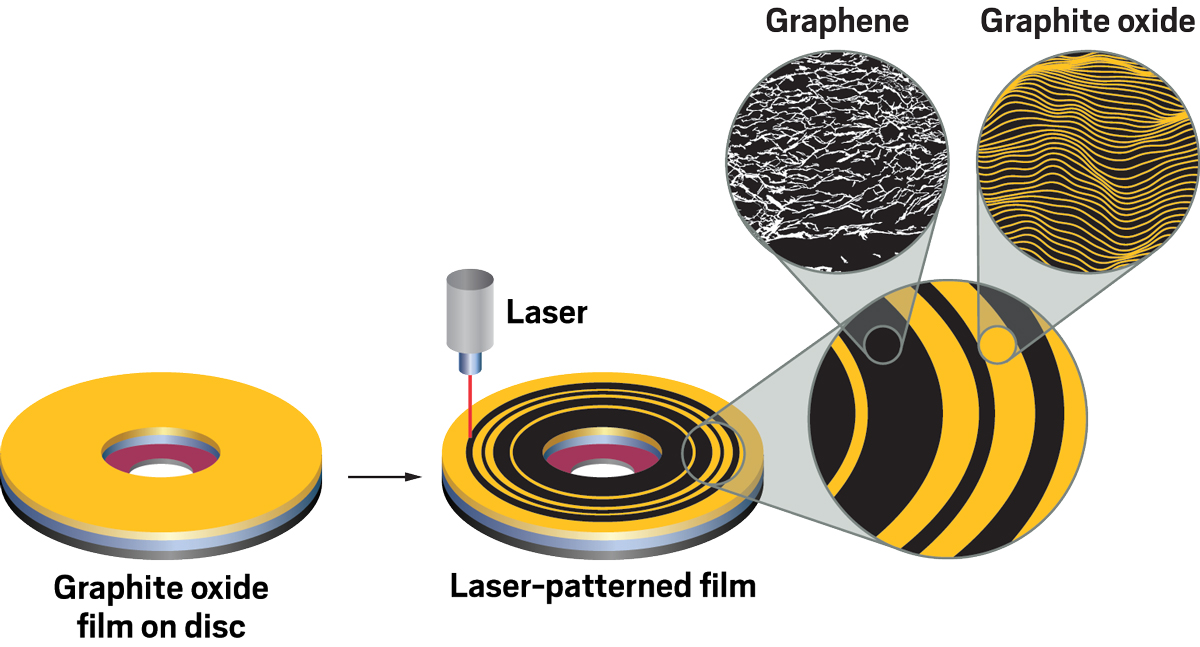
One of El-Kady’s eureka moments came when he was a grad student studying ways of using light-driven reactions to prepare graphene. He found that shining laser light on graphite oxide—a common, low-cost starting material often called graphene oxide—quickly converted the material to graphene, and he could draw graphene patterns with the fine laser beam. The product’s structure surprised him. Sheets of graphite oxide neatly stack like a thin ream of printer paper. But the laser-made graphene sheets crumpled in a way that formed a porous, 3D network.
Intrigued, El-Kady studied the process and determined that light absorption by graphite oxide generates heat and triggers photoreactions that pull oxygen atoms off the material, mostly in the form of carbon dioxide gas. The gas forces its way through the sheets, spontaneously crumpling them and forming an open, 3D structure with exposed graphene surfaces. Other methods for making graphene from graphite oxide form flakes with low internal surface areas because the sheets remain tightly stacked.
The porous graphene made with El-Kady’s method proved to be a great electrical conductor, so he knew he had an excellent charge-storage material for a battery. Also, the porous graphene’s 3D structure could make intimate contact with the charge-carrying electrolyte solution at the heart of batteries and supercapacitors, allowing for greater charge transport and storage. He quickly figured out how to use a low-power commercial laser to form intricate patterns of miniature, flexible supercapacitors and showed that they outperform standard ones made with activated carbon.
“My life was very different after that study was published,” he says. That work led to commercial inquiries, media attention, and follow-up projects on flexible, wearable, and implantable power sources, he says. It also led him to start Nanotech Energy, which raised $100 million to commercialize these laboratory advances and recently began selling conductive inks and other graphene-based products.
One of El-Kady’s main focuses now is Nanotech Energy’s Li-ion battery, which doesn’t burst into flames when punctured or short-circuited. Most of the details are proprietary, but El-Kady notes that the high-performance batteries are unique in that their electrodes are made with the company’s 3D graphene, the film that separates the electrodes remains stable at high temperatures, and the flammable organic electrolyte solution found in conventional batteries was replaced with a liquid that doesn’t ignite, even when exposed to a blowtorch.
Vitals
Current affiliation: Nanotech Energy and University of California, Los Angeles
Age: 39
PhD alma mater: University of California, Los Angeles
Hometown: Giza, Egypt
If I were an element, I'd be: “Carbon. Life on this planet wouldn’t be possible without carbon. It is the main element in proteins, DNA, and other biomolecules that are essential for all living forms. I want to have this impact in my community.”
My alternate-universe career: “Historian. I am obsessed with history, especially the history of science.”
See the Talented 12 present their work at a virtual symposium Sept. 19, 20, and 21. Register for free at cenm.ag/t12symposium.
|
|
Maher El-Kady is on a mission to reduce the world’s dependence on fossil fuels. To do so, he wants to build what he calls a “wonder battery” to power unprecedented numbers of electric vehicles.
Vitals
▸ Current affiliation: Nanotech Energy and University of California, Los Angeles
▸ Age: 39
▸ PhD alma mater: University of California, Los Angeles
▸ Hometown: Giza, Egypt
▸ If I were an element, I’d be: “Carbon. Life on this planet wouldn’t be possible without carbon. It is the main element in proteins, DNA, and other biomolecules that are essential for all living forms. I want to have this impact in my community.”
▸ My alternate-universe career: “Historian. I am obsessed with history, especially the history of science.”
“To speed up adoption of electric cars, we need batteries that hold enough charge to provide a long driving range, can be recharged quickly, and have a long life,” he says. “But they have to be safe—really safe—and affordable too.”
El-Kady thinks the key to making batteries with those specifications is a unique form of graphene and a simple way of synthesizing it. He discovered this process as a chemistry graduate student at the University of California, Los Angeles, where he now holds a position as a research professor. And today he’s making lithium-ion batteries with the material at Nanotech Energy, a start-up he cofounded and where he serves as chief technology officer.
“Maher is incredibly productive and an unbelievably good scientist—the best grad student I have ever had,” UCLA’s Richard B. Kaner says. Kaner notes that El-Kady has already coauthored nearly 200 patents and patent applications and published roughly 60 papers. He’s doing a top-notch job managing a team of 40 people, says Kaner, who serves as chair of Nanotech Energy’s scientific advisory board. “He’s mild mannered and the most creative guy you’ll ever meet.”
One of El-Kady’s eureka moments came when he was a grad student studying ways of using light-driven reactions to prepare graphene. He found that shining laser light on graphite oxide—a common, low-cost starting material often called graphene oxide—quickly converted the material to graphene, and he could draw graphene patterns with the fine laser beam. The product’s structure surprised him. Sheets of graphite oxide neatly stack like a thin ream of printer paper. But the laser-made graphene sheets crumpled in a way that formed a porous, 3D network.
Intrigued, El-Kady studied the process and determined that light absorption by graphite oxide generates heat and triggers photoreactions that pull oxygen atoms off the material, mostly in the form of carbon dioxide gas. The gas forces its way through the sheets, spontaneously crumpling them and forming an open, 3D structure with exposed graphene surfaces. Other methods for making graphene from graphite oxide form flakes with low internal surface areas because the sheets remain tightly stacked.
The porous graphene made with El-Kady’s method proved to be a great electrical conductor, so he knew he had an excellent charge-storage material for a battery. Also, the porous graphene’s 3D structure could make intimate contact with the charge-carrying electrolyte solution at the heart of batteries and supercapacitors, allowing for greater charge transport and storage. He quickly figured out how to use a low-power commercial laser to form intricate patterns of miniature, flexible supercapacitors and showed that they outperform standard ones made with activated carbon.
“My life was very different after that study was published,” he says. That work led to commercial inquiries, media attention, and follow-up projects on flexible, wearable, and implantable power sources, he says. It also led him to start Nanotech Energy, which raised $100 million to commercialize these laboratory advances and recently began selling conductive inks and other graphene-based products.
One of El-Kady’s main focuses now is Nanotech Energy’s Li-ion battery, which doesn’t burst into flames when punctured or short-circuited. Most of the details are proprietary, but El-Kady notes that the high-performance batteries are unique in that their electrodes are made with the company’s 3D graphene, the film that separates the electrodes remains stable at high temperatures, and the flammable organic electrolyte solution found in conventional batteries was replaced with a liquid that doesn’t ignite, even when exposed to a blowtorch.



















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter