Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Gene Editing
Alexis Komor
This base-editing ace develops tools to decipher how variations in our DNA might affect health
by Celia Henry Arnaud, special to C&EN
July 15, 2022
| A version of this story appeared in
Volume 100, Issue 25

Credit: Will Ludwig/C&EN/Tim Peacock/Michelle Fredericks
Our genomes—the vast collections of DNA that provide our underlying biochemical code—differ from person to person in millions of ways. But we know how only a fraction of those variations in DNA sequences affect health—for example, giving some people genetically low cholesterol or harboring a mutation that could lead to a deadly cancer. Alexis Komor thinks there’s much more we can learn from the differences in our genomes.
“Over 99% of the genetic variants that we’ve identified through sequencing, we have no idea what they actually do, how they’re impacting our health, whether positively or negatively or neutrally,” Komor says.
Advertisement
Komor, an assistant professor at the University of California San Diego, uses techniques she developed as a postdoc to introduce targeted changes to DNA in lab animals and cells and studies how those changes affect biological systems.
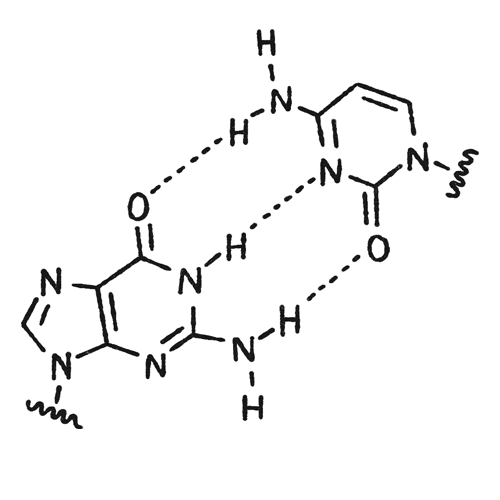
Throughout her career, Komor has used a molecular approach to get a detailed understanding of DNA. As a graduate student with Jacqueline K. Barton at the California Institute of Technology, Komor synthesized molecules that target DNA base mismatches, which often result in cancer-causing mutations.
“In my lab, she worked on designing molecules that bound preferentially to DNA mismatches as a first step toward designing new selective anticancer agents,” Barton says. “Her generation of molecules took us a whole quantum step further in terms of potency and selectivity.”
Komor then went to work as a postdoc with David R. Liu at Harvard University. “She’s one of the most determined people I’ve ever known. She is intellectually very rigorous and unforgiving in a really good way. Alexis is never tempted to trade off rigor for convenience,” Liu says. “As a result, she’s a brilliant scientist.”
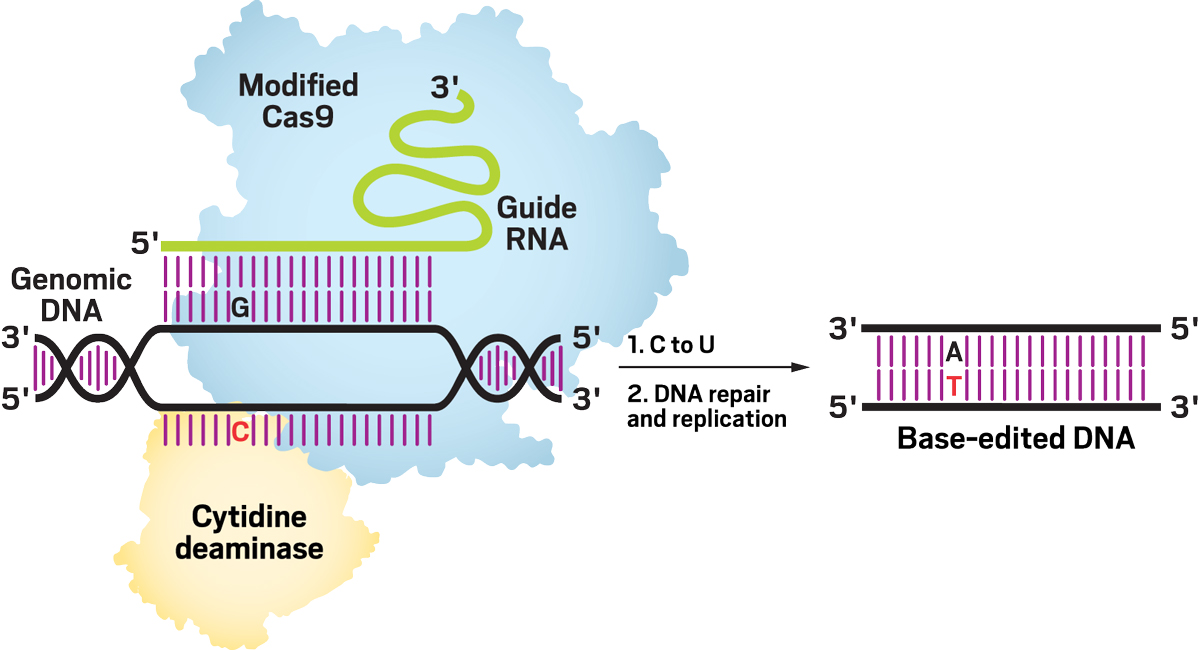
In Liu’s lab, Komor developed the first DNA base editor—a tool that can target a desired location of the genome and chemically alter a specific base. It was an elegant idea, but it could have easily failed. Liu says that because of Komor’s rigor, it was clear the approach was going to work from the very first set of experiments.
Because of the therapeutic promise of DNA base editors, people have approached Liu about turning the sequencing gel from Komor’s first successful base-editing experiment into a nonfungible token, or NFT, he says. She agreed on the condition that any proceeds go to a charity supporting women in science or science education for underrepresented groups.
Now, at UC San Diego, Komor is combining her work in Barton’s and Liu’s labs to forge a new research direction. She’s using base editing to understand how mutations in the genes that code for our cells’ DNA-repair machinery can lead to disease. Komor is developing tools to introduce variants into DNA at will so she can study how they affect cells and animals.
Her team changes a DNA base and then watches to see what proteins the cell sends to deal with the resulting modified base pair. Sometimes proteins from one DNA-repair pathway appear; other times, proteins from multiple repair pathways work together. What the researchers have started to observe can get weird, Komor says. “It’s pretty exciting, actually.”
Vitals
Current affiliation: University of California San Diego
Age: 34
PhD alma mater: California Institute of Technology
Hometown: Orinda, California
If I were an element, I'd be: “Phosphorus. Besides it being a major building block of DNA (the most awesome molecule of all time), I consider it a quite adaptable element, as it can expand its octet in order to form additional bonds.”
My alternate-universe career: “I would own a small farm in the South of France and produce my own wine and cheese.”
See the Talented 12 present their work at a virtual symposium Sept. 19, 20, and 21. Register for free at cenm.ag/t12symposium.

|
|
Our genomes—the vast collections of DNA that provide our underlying biochemical code—differ from person to person in millions of ways. But we know how only a fraction of those variations in DNA sequences affect health—for example, giving some people genetically low cholesterol or harboring a mutation that could lead to a deadly cancer. Alexis Komor thinks there’s much more we can learn from the differences in our genomes.
Vitals
▸ Current affiliation: University of California San Diego
▸ Age: 34
▸ PhD alma mater: California Institute of Technology
▸ Hometown: Orinda, California
▸ If I were an element, I’d be: “Phosphorus. Besides it being a major building block of DNA (the most awesome molecule of all time), I consider it a quite adaptable element, as it can expand its octet in order to form additional bonds.”
▸ My alternate-universe career: “I would own a small farm in the South of France and produce my own wine and cheese.”
“Over 99% of the genetic variants that we’ve identified through sequencing, we have no idea what they actually do, how they’re impacting our health, whether positively or negatively or neutrally,” Komor says.
Komor, an assistant professor at the University of California San Diego, uses techniques she developed as a postdoc to introduce targeted changes to DNA in lab animals and cells and studies how those changes affect biological systems.
Throughout her career, Komor has used a molecular approach to get a detailed understanding of DNA. As a graduate student with Jacqueline K. Barton at the California Institute of Technology, Komor synthesized molecules that target DNA base mismatches, which often result in cancer-causing mutations.
“In my lab, she worked on designing molecules that bound preferentially to DNA mismatches as a first step toward designing new selective anticancer agents,” Barton says. “Her generation of molecules took us a whole quantum step further in terms of potency and selectivity.”
Komor then went to work as a postdoc with David R. Liu at Harvard University. “She’s one of the most determined people I’ve ever known. She is intellectually very rigorous and unforgiving in a really good way. Alexis is never tempted to trade off rigor for convenience,” Liu says. “As a result, she’s a brilliant scientist.”
In Liu’s lab, Komor developed the first DNA base editor—a tool that can target a desired location of the genome and chemically alter a specific base. It was an elegant idea, but it could have easily failed. Liu says that because of Komor’s rigor, it was clear the approach was going to work from the very first set of experiments.
Because of the therapeutic promise of DNA base editors, people have approached Liu about turning the sequencing gel from Komor’s first successful base-editing experiment into a nonfungible token, or NFT, he says. She agreed on the condition that any proceeds go to a charity supporting women in science or science education for underrepresented groups.
Now, at UC San Diego, Komor is combining her work in Barton’s and Liu’s labs to forge a new research direction. She’s using base editing to understand how mutations in the genes that code for our cells’ DNA-repair machinery can lead to disease. Komor is developing tools to introduce variants into DNA at will so she can study how they affect cells and animals.
Her team changes a DNA base and then watches to see what proteins the cell sends to deal with the resulting modified base pair. Sometimes proteins from one DNA-repair pathway appear; other times, proteins from multiple repair pathways work together. What the researchers have started to observe can get weird, Komor says. “It’s pretty exciting, actually.”



















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter