Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Profiles
Movers And Shakers
Hispanic and Latino chemists you should know about
These pioneering scientists discovered new elements and helped develop the birth control pill
by Bibiana Campos-Seijo , Manny I. Fox Morone , Cheryl Hogue , Rachel Petkewich, special to C&EN; Ariana Remmel, special to C&EN; Shantal Riley, special to C&EN; and Attabey Rodríguez Benítez, special to C&EN
September 16, 2021

The contraceptive pill has been hailed as world-changing as the wheel, the printing press, and the internal combustion engine. Since its discovery in the 1950s it has liberated millions of women around the world—socially, sexually, and economically.
The birth control pill would not exist today without the work of Mexican chemical engineer Luis E. Miramontes. In 1951, Miramontes—a 26-year-old student at the time—synthesized norethindrone, the main component of the first effective contraceptive pill.
Miramontes is one of the scientists in this list of Hispanic and Latino chemists you should know about. This collection highlights the lives and careers of influential historical or recently deceased figures whose work has shaped our science and it follows collections that C&EN published to highlight the contributions of Black and LGBTQ+ chemists.
We hope these figures inspire future generations of chemical scientists—and not just Hispanic and Latino ones.
Take Mario Molina, the Mexican chemist who played a pivotal role in the discovery of the hole in the Antarctic ozone layer and who won a share of the 1995 Nobel Prize in Chemistry for work on elucidating the mechanisms of the formation and decomposition of ozone in the stratosphere.
And there are lesser-known Nobelists. Argentine physician and biochemist Luis F. Leloir received the 1970 Nobel Prize in Chemistry for his discovery of the metabolic pathways in sugars. César Milstein, also an Argentine biochemist, won a share of the 1984 Nobel Prize in Physiology or Medicine for his work on the chemistry of antibodies.
The list also includes scientists like Rebeca Gerschman, who was nominated for a Nobel Prize in Chemistry but died before she could receive it. She discovered that free radicals can cause oxygen toxicity and cell death. And we feature an element discoverer: Andrés Manuel del Río, the Spanish Mexican scientist who found vanadium in a piece of lead ore in 1801 but was not credited with the discovery at the time.
Ultimately, whether they are known or not, whether they’ve received accolades or not, they are trailblazing researchers, mentors, and educators that we should know about.
We will keep updating this collection of outstanding historic and recently deceased Hispanic and Latino chemists. Please share your nominees in this survey: cenm.ag/latinochemsurvey.
—Bibiana Campos Seijo
Oswaldo Luiz Alves
by Attabey Rodríguez Benítez

Oswaldo Luiz Alves was among the first Brazilian scientists to develop, research, and teach nanotechnology. He worked at the Laboratory of Synthesis of Nanostructures and Interaction with Biosystems, and he founded the Solid State Chemistry Laboratory at the University of Campinas, where he was a professor of chemistry.
Alves was born in São Paulo and was drawn to chemistry during after-school science camps in his adolescence. With a state fellowship, Alves took technical industrial chemistry courses and then finished his bachelor’s in industrial chemistry in 1973 at the public University of Campinas. At the same time that he began his doctorate at the university, he was hired there as a professor. But when he did his postgraduate studies in France, he “got contaminated,” as he called it, with solid-state chemistry. He then took this knowledge back to Brazil to found a solid-state chemistry laboratory. There he researched 2-D materials for electronics like glasses doped with quantum dots, and he filed patents, including one related to drug delivery using silica nanoparticles.
After more than 40 years of teaching, he died suddenly in July 2021. He taught more than 50 master’s and doctoral students who carry on his legacy.
Sara Borrell Ruiz
by Attabey Rodríguez Benítez

Sara Borrell Ruiz was one of the first experts on sex hormones in Spain. She worked with the team that was developing the first oral contraceptive and was the director of the steroid division at the Gregorio Marañón Health Research Institute, headquartered in Madrid.
While in Spain working on her bachelor’s in pharmacy in 1933, she became interested in water sanitization and did her graduate studies in that field. In 1944, Borrell Ruiz earned her doctorate for her study of the waters of the Tagus River.
Two years later, she received a postdoctoral fellowship to study milk composition and conservation in Scotland. She hoped to address supply issues the British suffered after the war. She focused on the mechanism behind the browning of milk, which was not understood at that time. Borrell Ruiz found that a reaction between glucosamine and a base caused browning (Nature 1952, DOI: 10.1038/1691097a0).
This work with milk eventually led Borrell Ruiz to her interest in hormones, which would sustain the rest of her career. She had read that some cows produced more milk when they ate in fresh pastures, and researchers had recently determined that grass had estrogenic compounds.
Borrell Ruiz traveled to the US to research estrogen and other sex hormones in the 1950s, 10 years before the birth control pill was approved in the country. She studied the hormonal composition of urine from pregnant women, but her results were not published. Afterward, she returned to Spain and became a professor of neuroendocrinology at the Cajal Institute and joined the Gregorio Marañón Health Research Institute, which was built in honor of her previous mentor. There she researched the metabolism of adrenal hormones in different animals to determine their effect until she retired in 1988 after 43 years.
Andrés Manuel del Río
by Shantal Riley
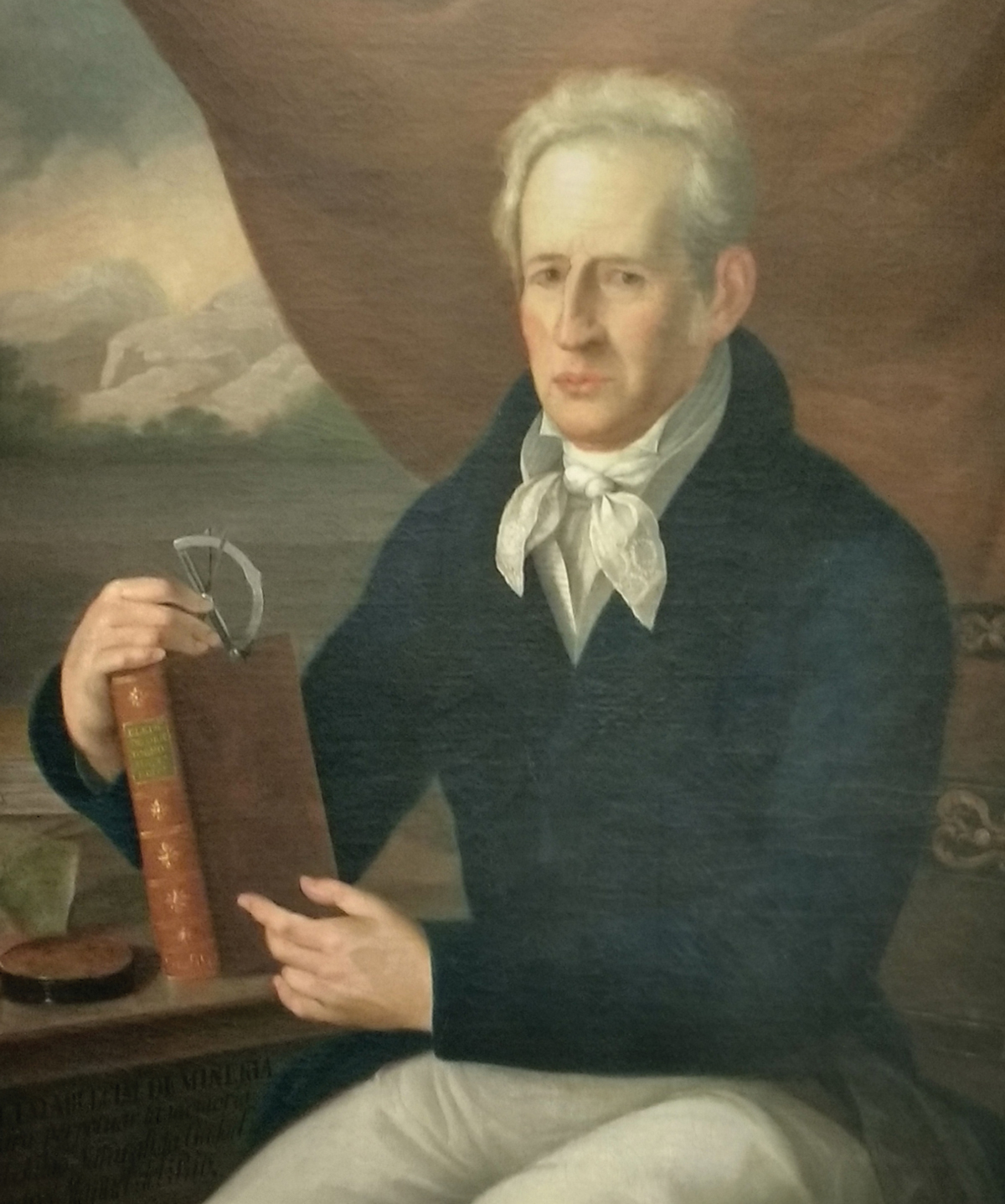
In 1801, Spanish-born chemist Andrés Manuel del Río discovered the only element to be unearthed in Mexico. But it took 30 years before he was credited for finding vanadium, a silvery transition metal and number 23 on the periodic table.
Del Río was working as a professor of mineralogy at the School of Mines in Mexico City when he found the metal in a piece of lead ore from Zimapán, Mexico. He named it “erythronium,” from the ancient Greek word erythros, meaning red. He was inspired by the red color formed by its salts.
He later sent the sample to Paris, where chemist Hippolyte-Victor Collet-Descotils mistakenly concluded that it was chromium and not a new metal as del Río had claimed.
In 1830, chemist Nils Gabriel Sefström rediscovered the element in iron ore from Sweden. He named it “vanadium” after Vanadis, the Norse goddess of love and beauty. Soon after, German chemist Friedrich Wöhler analyzed del Río’s erythronium and confirmed it contained vanadium. But del Río never received proper credit for his discovery while he lived.
Vanadium’s resistance to heat and corrosion led to its use in WWI artillery and the Ford Model T engine. Today, vanadium is used in alloys that build tools, engines, and machinery.
Del Río lived in Philadelphia for a time after Spanish-born people were expelled from Mexico following the war of independence, but he returned to the country in 1834. He is the author of Elementos de Orictognosía, the first textbook on mineralogy published in the Americas. Each year, the Mexican Chemical Society bestows an award in his name to chemists who have made extraordinary contributions to science.
Rebeca Gerschman
by Attabey Rodríguez Benítez

Argentine biochemist Rebeca Gerschman was the first scientist to propose that free radicals can cause oxygen toxicity and cell death (Science 1954, DOI: 10.1126/science.119.3097.623). The connection between free radicals and aging, first proposed by Gerschman in 1954, is still being explored by scientists.
Gerschman earned her PhD at the University of Buenos Aires in 1937. During her graduate studies, she developed a method, later known as the Gerschman-Marenzi method, to study potassium in the blood. She did postdoctoral work at the University of Rochester, where she continued studying potassium. But her mentor was also interested in respiration, and she followed that interest, beginning to study oxidative stress.
This was when Gerschman started to study the effects of gases, like oxygen, in animals, inspired by an observation that the skin of fighter pilots was aging at faster rates than non-pilot folks. Fighter pilots are exposed to about the same radiation as a tanning bed. At increasing altitudes, oxygen concentrations can go from the usual 21% up to 100%. Gerschman surmised that a lot of oxygen and radiation could generate radicals that lead to faster skin aging in the pilots.
In the 1980s, she was nominated for a Nobel Prize for her contribution to the study of free radicals, but she died before she could receive the award.
Luis F. Leloir
by Manny Morone

Pursuing science during a tumultuous time in Argentina’s history, when the government was hostile to academic work, Luis F. Leloir made foundational discoveries about glucose metabolism. Leloir was born in 1906 in Paris to an Argentine mother and first came to the country as a toddler. He would go on to become the first Latin American to win the Nobel Prize in Chemistry.
Leloir started his scientific career in 1932 as a medical doctor. Unhappy with the tools available to doctors at the time, Leloir left clinical medicine after 2 years and began working in the medical research labs at the University of Buenos Aires (UBA). He spent a year at the University of Cambridge building expertise in biochemical research and in 1937 returned to UBA, where he continued research in the university’s physiology department and eventually started teaching students.
Leloir’s research was interrupted in 1943 when Argentina’s constitutional government was overthrown by the military. Thousands of academics were fired under military rule, including Leloir’s longtime mentor, Bernardo Houssay. Houssay, a future Nobelist himself, was dismissed after signing an anti-Nazi letter of protest. Leloir once again went abroad, continuing his research in the US and England.
He returned to Buenos Aires in 1947 and became the founding director of the privately funded Campomar Foundation Institute for Biochemical Research (now called the Leloir Institute Foundation). Several independent research institutes had been popping up in Buenos Aires, often run inside converted houses and staffed by academics who had been fired from public institutions.
Here, Leloir probed the enzymatic pathway that converts the sugar galactose to glucose in cells. His group identified a major player: the nucleotide uridine diphosphate, which connects to sugar molecules and allows them to be digested by enzymes and used as energy.
Throughout the 1950s, Leloir and his colleagues studied how nucleotide sugars affect energy storage and the breakdown of complex polysaccharides. The team discovered that glucose could be stored in the long sugar chains attached to the protein glycogen only after glucose formed the intermediate uridine diphosphate glucose. This discovery and others about nucleotide sugars’ role in metabolism earned Leloir the Nobel Prize in Chemistry in 1970.
Leloir died in 1987, still an active researcher. In 2001, the Campomar Institute was renamed in his honor.
César Milstein
by Ariana Remmel

César Milstein was an Argentine Nobel laureate who studied enzymology and immunology. Milstein, born in 1927, is best remembered for his research in antibody production.
In his early career, Milstein made significant contributions to the understanding of enzyme kinetics and reaction mechanisms. In 1961, he accepted a position as a division head at the National Institute of Microbiology in Buenos Aires to continue this work.
In 1963, Milstein resigned from his post because of what he described as “political persecution of liberal intellectuals and scientists.” He moved to Cambridge, England, to join the lab of protein chemist Frederick Sanger, an early mentor and longtime collaborator, who suggested that Milstein shift his research focus to immunology.
Milstein became interested in the diversity of antibodies, which are proteins that immune cells use to target and eliminate disease-causing agents. But the difficulty of making antibodies in the lab created a major obstacle to designing experiments that might reveal the underlying mechanisms in immune biochemistry.
While investigating the relationship between antibody diversity and production, Milstein and collaborator Georges J. F. Köhler developed a technique to fuse two cell lines: one cancerous and another that produces a specific antibody. The result was a hybrid cell line that grows easily in lab cultures and churns out monoclonal antibodies in large quantities (Nature 1975, DOI: 10.1038/256495a0). Milstein and Köhler each got one-third of the 1984 Nobel Prize in Physiology or Medicine for this discovery.
The newfound accessibility of these proteins revolutionized the study of immunology and human medicine, and Milstein spent much of his later career developing new applications for monoclonal antibodies in the lab. He died in 2002.
Luis Miramontes
by Rachel Petkewich

C&EN published a version of this profile on Sept. 11, 2006
Luis Miramontes co-invented a compound used in birth control pills. Born in Tepic, Mexico, Miramontes earned a BS in chemical engineering in 1954 from the National Autonomous University of Mexico (UNAM).
Miramontes started his career as a researcher in organic chemistry at the Institute of Chemistry at UNAM. He joined the pharmaceutical company Syntex in Mexico City, where he worked on the synthesis of various compounds related to steroids.
On Oct. 15, 1951, Miramontes, then 26, synthesized norethindrone, which would become the active compound base of the first synthetic oral contraceptive pill. He received the patent on the compound with Carl Djerassi and George Rosenkranz.
Miramontes continued as a researcher in organic chemistry and taught in several prestigious Mexican universities. Among his awards, he received the Andrés Manuel del Río prize, the top chemistry prize in Mexico. In 2005, the Mexican Academy of Sciences nominated Miramontes’s work on the oral contraceptive pill as the most important Mexican contribution to world science. He died in 2004.
Mario Molina
by Cheryl Hogue

C&EN published a version of this profile on Oct. 9, 2020.
As a boy growing up in Mexico City, Mario Molina played with a chemistry set. As an adult, he shared the 1995 Nobel Prize in Chemistry for work on understanding the formation and decomposition of ozone in the stratosphere.
His research helped pave the way to a landmark treaty to protect stratospheric ozone, the 1987 Montreal Protocol on Substances That Deplete the Ozone Layer.
In 1974, Molina was a postdoctoral researcher under F. Sherwood Rowland at the University of California, Irvine, when the two published a groundbreaking paper in Nature (DOI: 10.1038/249810a0). They hypothesized that chlorofluorocarbons (CFCs), synthetic gases then widely used as refrigerants and aerosol propellants, could deplete stratospheric ozone, which absorbs most of the sun’s ultraviolet light and makes life on Earth possible.
Chemical manufacturers and aerosol producers scoffed at the idea. But other scientists confirmed and built on Molina and Rowland’s work.
Molina shared the Nobel Prize with Rowland and Dutch atmospheric chemist Paul J. Crutzen. Molina received the US Presidential Medal of Freedom in 2013.
Molina was active in environmental policy, urging world leaders to address climate change and working to establish policies to improve air quality in the world’s megacities.
He earned a doctorate in physical chemistry at the University of California, Berkeley, and worked at the Jet Propulsion Laboratory at the California Institute of Technology. Molina was a professor at the Massachusetts Institute of Technology and the University of California San Diego. He founded the Mario Molina Center for Strategic Studies on Energy and the Environment in Mexico City.
Molina died in 2020 at age 77.
Sarah Stewart
by Ariana Remmel

Children today routinely receive vaccines against human papillomavirus, which can cause cervical cancer and other diseases. This lifesaving treatment is thanks in part to the work of Sarah Stewart, who was among the first to demonstrate a link between viruses and cancer.
Stewart was born in 1905 in Tecalitlán, Mexico, and moved to the US early in her childhood. She pursued both master’s and doctoral degrees in microbiology, which led to her interest in viruses and human disease.
At the time, there was already evidence that some viruses might be associated with tumor growth. But most researchers in the mid-20th century didn’t think the link was worth investigating. When Stewart submitted her first application to look for cancer-causing viruses in 1944, the US National Institutes of Health denied her funding request, stating that the project lacked merit and that her background in microbiology made her unqualified to study human medicine.
But Stewart was relentless. She took a teaching position at Georgetown University, where she taught microbiology at the School of Medicine. At the time, only men were allowed to enroll in the medical school, but Stewart’s faculty position allowed her to audit classes alongside the male students. When the school finally allowed women to matriculate, Stewart became the first woman to earn a medical degree from Georgetown in 1949.
With doctoral and medical degrees in hand, Stewart joined the lab of Bernice Eddy in 1951 at the NIH, and the pair began many years of careful experimentation, searching for cancer-causing viruses. Finally, in 1958, Stewart and Eddy became the first scientists to establish a definitive link between tumor formation and a newly described virus that they called SE (for Stewart Eddy) polyomavirus (Proc. Soc. Exp. Biol. Med. 1958, DOI: 10.3181/00379727-98-24205). Stewart and Eddy’s discoveries place them squarely among the founders in the field of viral oncology.
Stewart was nominated for the Nobel Prize twice, and her achievements were recognized in other ways during her lifetime. In 1965, President Lyndon B. Johnson presented her with the Federal Women’s Award for her contributions to cancer research. Stewart continued her research in viral oncology for the rest of her career until she died of cancer in 1976.
Evangelina Villegas
by Ariana Remmel

Mexican biochemist Evangelina Villegas is best remembered for her contributions to cereal and nutritional science, especially for her work with maize. She lived from 1924 to 2017 and spent most of her life in Mexico City, where she was born.
Villegas’s interest in maize as a culturally and economically important crop in her home country and her affinity for science led her to earn a PhD in cereal chemistry and breeding from North Dakota State University.
In 1967, she joined the International Maize and Wheat Improvement Center in Mexico, where she worked to combat global malnutrition. She was soon joined by collaborator Surinder Vasal, and together they set their sights on maize (corn), a staple crop that feeds millions of people around the world.
Despite the crop’s caloric importance, most varieties of maize are low in protein, especially the essential amino acids lysine and tryptophan. Without the benefit of a varied diet, people who subsist predominantly on maize, which is common in parts of Africa, Asia, and South America, are at higher risk of malnourishment and associated illnesses. The protein deficiency of these diets is especially dangerous for infants, who are often weaned with maize. Villegas and Vasal hoped to combine biochemical and plant breeding techniques to engineer a protein-rich maize with a palatable flavor and texture that could fill the nutritional needs of maize-dependent communities.
After more than a decade of work, the pair developed a variety called quality protein maize (QPM). QPM not only has a higher overall protein content than conventional maize but also contains nearly double the amount of lysine and tryptophan.
QPM was soon grown in parts of Africa, where it was shown to improve the health of young children who had malnutrition. Its use has since expanded to countries in South America and Southeast Asia. Villegas and Vasal shared the 2000 World Food Prize for their contributions to global nutrition, with Villegas becoming the first woman to earn the award. In the same year, Villegas was presented with the Woman of the Year award by the Mexican Women’s Association. She went on to work as a consultant to help share the research and applications of QPM around the world.




Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter