CHEMISTRY ON


Advances in materials science and chemistry bring ice hockey a long way from its 19th-century roots.
Illustration by Alexander Wells
A Halifax, Nova Scotia-born lawyer and engineer named James Creighton placed that notice in the paper. He and his friends had honed their hockey skills on Canada’s frozen rivers and ponds, fighting to control a skittering lacrosse ball in rowdy games with negligible rules and flexible team sizes. With the exhibition game at the Victoria Skating Rink, Creighton saw the opportunity to take the game indoors and lay down some rules.
Teams were limited to nine members and, to prevent damage to spectators or venue, the players substituted a block of wood (some reports noted that it was circular; others said it was square) for the livelier lacrosse ball.
With rules now in hand, two teams of young men—mostly students from McGill University—raced across the ice on blades mounted to leather boots. They thwacked their wooden sticks against the frozen surface and each other, as opposing players battled to gain possession of the puck. Though they played indoors, the competitors enjoyed a smooth, frozen rink, courtesy of Montreal’s cold late-winter days and nights.
Nowadays, hockey is a juggernaut. Tens of thousands of spectators watch players, decked out in high-tech gear developed through material and chemical innovation, race to control a circular puck using sticks made from high-tech composites. One stadium regular is chemist and superfan Anthony M. Noce, vice president at Tetra Tech, an international consulting engineering firm, and chair of the ACS Committee on Environmental Improvement.
Chemistry is everywhere in ice hockey, says Noce, who has witnessed decades of change and innovation over his lifetime of fandom. It has been the game’s enduring teammate, supplying novel materials, coatings, and refrigerants to help players and play evolve.
Ice-capades
Given Canada’s bountiful lakes and rivers, it's no surprise that Creighton and his friends took advantage of the natural freeze to enjoy winter sports. In a modern stadium, though, skilled technicians combine under-ice refrigeration systems, environmental controls, cutting-edge refrigerants, and ponderous resurfacing vehicles to produce and manage the ice.
In its liquid state, water molecules jostle around as the bonds between them constantly break and re-form. As water cools, these molecules slow and the bonds stabilize. Around 0 °C, they become rigid, initiating the change from liquid to solid.
In nature, frozen water takes on an array of irregular structures, including snow, icebergs, hail, and icicles, depending on conditions. That variability won’t do for a rink, which must have a level, low-friction surface perfect for a fast puck and efficient skating. Making that takes more than just cooling down a pool of water. Rinks use a sophisticated system that sprays ultrathin layers of water on a bed of sand or concrete embedded with cooling pipes filled with refrigerant. The layers are frozen, one-by-one, stacking up to a smooth playing surface measuring between one inch and one-and-a-half inches thick.
Even the water itself is engineered. “Some arenas, including the NHL buildings, process their water in special ways,” says Kelly McMillen, an engineer at Zamboni Co. The rinks want to control the amounts of total dissolved solids in the water, such as minerals and salts, and use processes such as deionization and filtration to achieve the desired levels. Harvested rainwater, used at rinks such as the Abbotsford Entertainment and Sports Centre in Canada, has a low mineral content, whereas the mineral content of water from lakes or the ground varies widely. The higher the number of mineral particles in the ice, the greater the friction between surface and blade; more friction equals less speed.
“The most common water treatment method in the ice rink industry is reverse osmosis to control water hardness,” explains Jeff Theiler, chief operating officer at the U.S. Ice Rink Association. “The main impurities that we want to minimize are calcium and magnesium.” He adds that the harder water lowers water’s freezing temperature and creates a less dense ice, which lead to higher costs.
No matter how good the water and resulting ice is, it won't stay smooth for long. “Over the course of play, the skates are cutting into the ice,” explains Noce, who has been fascinated by the ice-making process since he was a kid. “You're scarring it.”
Rutted ice slows play and needs to be resurfaced. Originally, to clear the shavings left by passing skates, workers resurfaced the ice with shovels, then covered it with fresh water from a hose. This filled in the grooves and marks left by the charging skaters, making the top layer of ice smooth again. That whole process, then lasting over an hour, is now repeated four times per NHL game and is carried out by modern machines that repair the ice in minutes.
Frank Zamboni gets the thanks for that. An engineer, Zamboni, patented his eponymous vehicle in 1949. The Zamboni® machine creates a smooth surface in three steps that all happen as the machine glides over the ice. First, it shaves the ice and collects the shavings. Next, it washes the top layer of the ice and vacuums up the dirty water. Finally, it lays down a fresh layer of warm water and smooths it out. (Warm water contains fewer impurities—which produce distorted ice with greater friction—than cold water.)
The much-loved Zamboni® machine has taken on the role of mascot in many arenas, McMillen says, adding that the contrast between the machine's slow movements and the speed of hockey appeals to spectators. “It's amazing to see people mesmerized by the resurfacing process,” he says.
Protective elements
Nineteenth-century hockey players wore casual shorts and sweaters, leaving them vulnerable to injury. Goaltenders, who frequently dive onto and slide across the ice to protect the net, were particularly exposed. Thankfully, chemistry has improved their lot.
Ryan J. Frayne, a hockey player and researcher based at the Dalhousie University, studies the effects of goaltender protective equipment on athlete safety and performance. One of the biggest innovations in recent years has been the implementation of molded foam cores used to stabilize the structure of leg pads.
The foam core improves the pad’s longevity and performance “because it permits predictable rebounds and provides a stable surface for goaltenders when they are performing butterfly movements,” Frayne says. In other words, a goaltender will have a good idea of where the puck will go once it bounces off his pads and a firm foundation when he drops to his knees and splays his lower legs to protect the net.
To combat high-velocity shots, some manufacturers now use cutting-edge foams in their protective equipment, Frayne explains. For example, D3O is a U.K. company that produces flexible foams and elastomers for impact protection and shock absorption. Their hockey pad foams are based on polymer blends constructed from molecules whose properties change under load. They stiffen when absorbing the energy of a high-velocity impact yet remain pliable when the impact is gentler. This lets the equipment bend with the hockey players’ movements and still provides a protective shell for hard blows from the puck.
Players also depend on helmets for safety. The shell of a modern helmet is usually made from vinyl nitrile, which disperses the force from an impact. Inside, liners made from vinyl nitrile foam or expanded polypropylene foam absorb energy and reduce the chances of injury.
While helmets are obligatory in a game, there is no requirement to wear full face protection. Many players, Noce explains, don't like the protective cages, since they obstruct vision, and some players even complain about the current visors. Still, Noce thinks novel materials, such as high-strength, clear thermoplastics, will solve the problem.
One solution to the visibility problem is the Lexan visor made of polycarbonate resin thermoplastic that can undergo extreme heat and significant deformation without cracking or breaking. According to the manufacturers, this makes it 250 times stronger than glass and 30 times stronger than acrylic.
“These visors have really opened things up,” Noce says. “If we can get the antifog technology down, whether by some coating or by a change in the material, I think you'll see a requirement for full-face visors in the future.“
Slide and glide
In 1875, Creighton and his team won their game wearing simple leather shoes perched atop metal blades. But these were far from being the first ice skates.
Early skates were made of animal bones attached to the feet by leather straps. Heavy bone blades were eventually replaced by short metal. From the 18th century onward, blades became longer as designers developed increased mechanical understanding of skating. Longer blades resulted in a greater contact area with the ice, keeping the blade from digging into the ice as much, which in turn means less resistance and faster, smoother skating.
Leather boots have given way to synthetic materials such as nylon, poly-paraphenylene terephthalamide (better known as Kevlar), and graphite. These materials make for a more durable, protective, and lighter skate.
In particular, poly-paraphenylene terephthalamide, a high-strength synthetic fiber, can be produced in different forms, such as sheets and ropes, and used in composites. It plays a role beyond skates, being central to the manufacture of sticks, protective gear, and even extra-tough socks.
Stick notes
Hockey sticks were once, as the name suggests, carved from wood, such as elm, birch, aspen, or ash. And just like his 19th-century predecessors, Noce earned his childhood hockey stripes playing with wooden sticks.
Wood, though, has a serious flaw: It breaks under pressure. “If I were a coach, the last thing I need is to have my defenseman wind up for a big shot and have a stick break,” Noce explains. Hockey sticks are now built from lightweight, more elastic composites that resist splitting. “The flex makes a huge difference in the speed and accuracy of shots,” Noce notes.
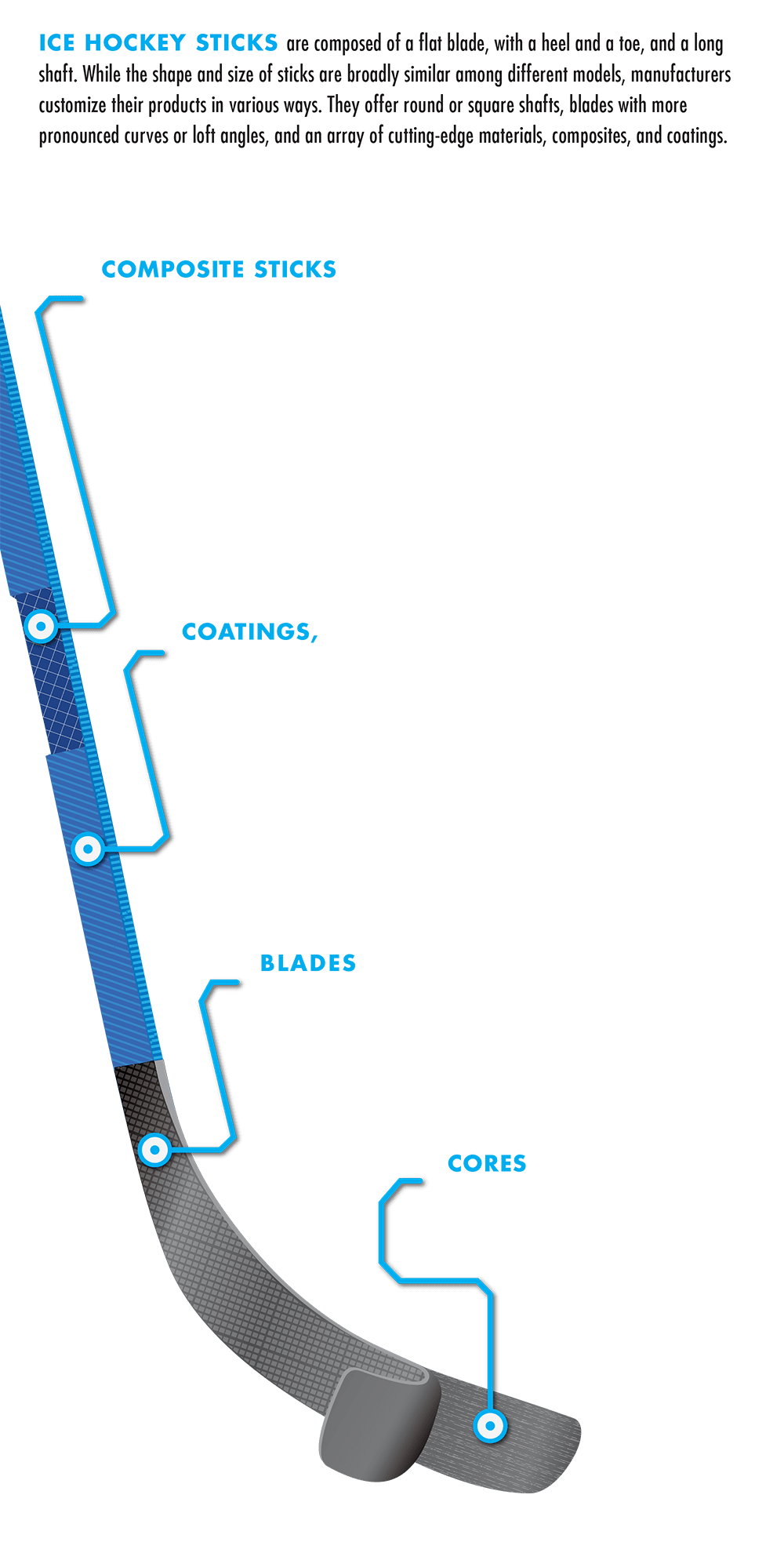
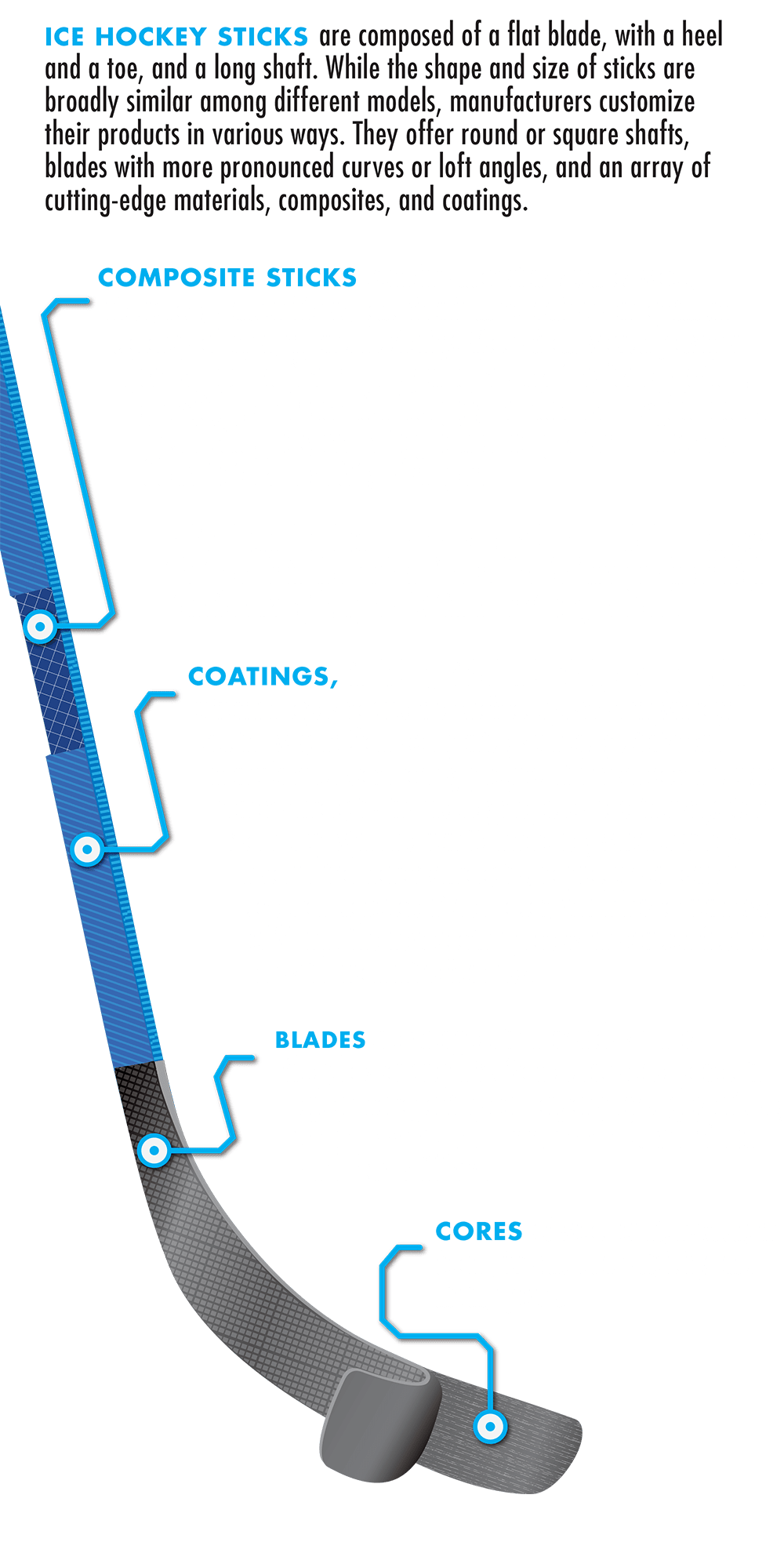
To make composite sticks, wood is combined with materials such as fiberglass, aluminum, titanium, and carbon fiber. The elastic properties of composite materials depend on the fiber material and the angle of the weave, as well as the geometry of the shaft itself.
Some stick blades are even coated in carbon nanoparticles. Coating blades with these nanoparticles reduces friction with water molecules and provides a smooth and hard surface, which can be sharpened and shined, by heavy polishing, to a mirror finish.
Puck-fection
When Creighton rounded up his friends to play that first game of indoor ice hockey, he introduced a new element that revolutionized the game. As the Montreal Gazette recapped the day after that first match:
“Hockey is played usually with a ball, but last night, in order that no accident should happen, a flat block of wood was used, so that it should slide along the ice without rising, and thus going among the spectators to their discomfort.”
While most gear has evolved beyond recognition, that small block was not unlike the disc-shaped puck players know today. Now, however, it is made of rubber rather than wood. Carbon is added to improve the rubber's resistance, while other additions, such as sulfur and antioxidants, can increase puck strength and hardness.
Pucks are often frozen before a game to make them less prone to bouncing. Cold pucks have a lower coefficient of friction and glide more efficiently; this also gives players more control over the puck’s speed and direction. To ensure fair play and consistency during NHL games, linesmen regularly replace used pucks with new frozen ones.
“Shins and heads were battered”
Accounts vary of how the first-ever indoor game ended. According to the Gazette, the event was a resounding success attended by a very large crowd.
The Kingston British Daily Whig, however, reported an event that will be familiar to any hockey fan: a fight. Some players got into a brawl with members of the local skating club, who were watching the game. The reporter noted, “Shins and heads were battered, benches smashed, and the lady spectators fled in confusion.”
Whatever the truth of the tale, should those Victoria Rink players and spectators miraculously find themselves at a modern hockey game, they would encounter some familiar trappings. They would see the tradition of the hockey fight endure, even if it's usually among the players and not the spectators. But much else would be utterly foreign: high-tech sticks, cushioned helmets, and Zamboni® machines. Despite these advances, it is the future of the humble puck, relatively unchanged since Creighton swapped a lacrosse ball for a block of wood, that gets 21st-century spectator Noce most excited.
“I want to see them put a microchip in the puck so we know when it crosses the goal line,” he says. “I would also like to see the tracking data from the pucks and see just how fast that thing is moving. I would love to geek out on some of that.”
Noce won't have long to wait. The NHL is testing a real-time data-tracking smart puck, which it hopes to introduce league-wide in the 2019–2020 season.
Precipitating the conversation:
A Perspective from Stefanie Kopchick, Global Market Manager, Opteon™ Stationary Refrigerants at The Chemours Company

Face-off! The puck drops. The players slap at it. A pass and then another. A stick is raised, and by the time the crack registers in the stands, the puck is at the back of the net. The siren sounds, but who can hear it over the roar?
It all happens so fast. That's the beauty of hockey—its speed. We have ice to thank for that. If the ice surface is not the right temperature, it can slow down the puck, the players, and the excitement. Controlling that ice requires a minor miracle of chemistry.
Ice plants running on Opteon™ refrigerants can produce ice surfaces that meet the exacting standards of professional hockey. And they do it without depleting the ozone layer, while significantly reducing global warming potential (GWP). The hydrofluoroolefin (HFO)-based refrigerants in the Opteon™ portfolio also provide rink operators with energy-efficient, nonflammable, and low toxicity solutions—reducing the cost and complexity of running and maintaining the rinks, compared to other refrigerant options.
The chemistry continues above the ice too. Chillers, also running on refrigerants, keep the air cool and dry. Improper temperature and humidity in the arena can distress the ice, slowing down players, not to mention bringing discomfort to the athletes and fans.
The magic of hockey extends far beyond the walls of professional arenas. Local rinks, which bring communities together for a fun day of skating or junior hockey, need to remain affordable. Anything that increases the costs of running a facility, though, may drive up rink fees, which can deter potential players. Case in point: R-22, a commonly used refrigerant in ice rinks. Environmental regulations are phasing out R-22, causing supply-and-demand imbalances and, ultimately, significant price fluctuations. This leaves many rink owners and operators grappling with big financial questions.
Fortunately, Opteon™ isn't just environmentally sustainable; it's a cost-effective, long-term solution. Opteon™ refrigerants can provide safe and affordable solutions, enabling rinks to retrofit existing equipment with minimal transition time—or even install new systems when needed.
Opteon™ low GWP products are emblematic of the advances chemistry continues to make. It, and other breakthroughs like it, provide more opportunities for innovative, sustainable technologies that protect the environment and the bottom line.
READ HOW CHEMOURS PRODUCTS HELP ENABLE WINTER SPORTS:
November 26: The Chemistry of Travel
December 17: The Chemistry of Dining
HOW CHEMISTRY ROCKS MUSIC FESTIVALS
PACKING THE RIGHT MOLECULES FOR THE GREAT OUTDOORS
HOW CHEMISTRY THRILLS IN AMUSEMENT PARKS
ABOUT SPONSORED CONTENT
Sponsored content is not written by and does not necessarily reflect the views of C&EN's editorial staff. It is authored by writers approved by the C&EN BrandLab and held to C&EN's editorial standards, with the intent of providing valuable information to C&EN readers. This sponsored content feature has been produced with funding support from The Chemours Company.