Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Sustainability
Kevin Barnett
Polymer pragmatist is creating economical biobased polymer building blocks
by Ryan Cross
August 14, 2020
| A version of this story appeared in
Volume 98, Issue 31

Credit: Courtesy of Merve Ozen (Barnett); Molview (structure); Shutterstock (corncobs)
Just because something is possible doesn’t mean it is practical. That’s a mantra that William Banholzer’s students know all too well. Banholzer, a former chief technology officer of Dow Chemical Company, teaches a class at the University of Wisconsin–Madison, where he trains his students to evaluate the economic successes and failures of chemical companies.
The course struck a chord with Kevin Barnett, who had recently joined the lab of renowned biofuels researcher George Huber. Barnett arrived at graduate school eager to combine his passion for the environment with his interest in chemical catalysis. But as Barnett soon learned, Banholzer is a vocal critic of the biofuels industry, where many scientifically ambitious ventures fail due to their economic infeasibilities.
Advertisement
So in Huber’s lab, Barnett began working on a Department of Energy–sponsored project to use renewable resources to make a high-value commodity chemical. The molecule, 1,5-pentanediol (1,5-PDO), is a building block of several polymers that are used to make a plethora of products including synthetic leather, high-performance paints, industrial coil coatings, adhesives, and more. Today, 1,5-PDO is made as a by-product from processing petroleum, and its relative rarity and high cost have limited its use.
In search of a cheaper, greener, and renewable source of the molecule, scientists have made 1,5-PDO from tetrahydrofurfuryl alcohol (THFA), a molecule derived from corncobs. Researchers discovered a catalytic reaction that creates 1,5-PDO in a single step by breaking the five-membered ring of THFA. “It was amazing chemistry,” Barnett says, but the catalysts it required were too expensive for wide adoption.
In Huber’s lab, Barnett sought a cheaper route to 1,5-PDO by dividing the single reaction into two simpler steps. “But no matter what I did, it wasn’t economical,” he says.
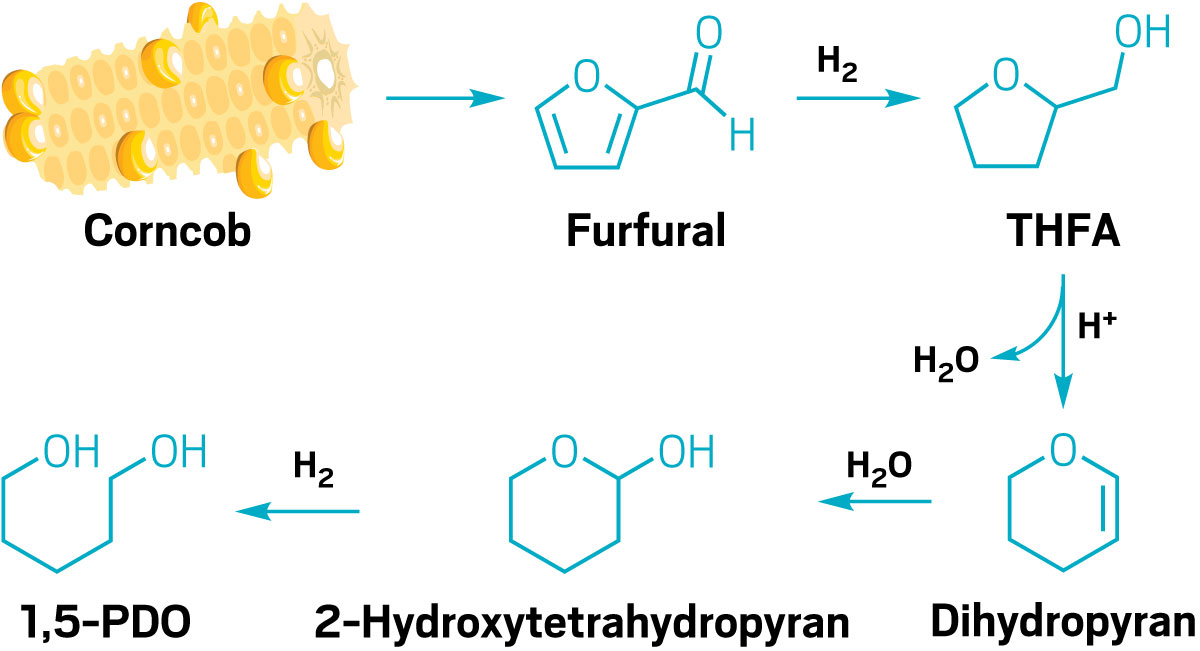
So he tried three steps.
“You might think that would be more costly,” he adds, but his elongated synthesis proved more efficient and selective at every step. Importantly, the penultimate step created an intermediate molecule with a ring that opened on its own—no costly catalyst needed.
Barnett spent the next 3 years optimizing the reaction. After graduating in 2018, he became the first employee, and CEO, of Pyran, a start-up that he and Huber cofounded to scale up and commercialize biobased 1,5-PDO. In the 2 years since, Barnett has conducted a market analysis, identified potential customers, pitched investors, hired the firm’s first two engineers, and scrutinized the spider web of products that currently use, or could use, 1,5-PDO. “Kevin did all the heavy lifting,” Huber says.
This year, Pyran plans to deliver its first 10–20 metric tons of product to its customers, priced at about $3,000 a metric ton—40% less than petroleum-derived 1,5-PDO sold by large chemical companies like BASF. Barnett estimates that the market for the molecule will be about $100 million–$200 million in a few years, although that figure could rise further in the future. Pyran is working with academic collaborators to explore making new polymers for 1,5-PDO, including biodegradable plastics—an application previously left untapped due to the molecule’s historically high cost.
While the success of biobased 1,5-PDO is no guarantee, Barnett’s pragmatism may help him navigate the start-up through a field littered with the remains of biobased chemical ventures whose economic compasses weren’t as finely tuned. And his practicality has even won over biobased fuel skeptic Banholzer, who joined Pyran’s board.
“It is a completely different animal,” Barnett says of his transition from laboratory scientist to budding chemical businessman. “But now that I am an entrepreneur, I don’t think there is ever any going back.”
Vitals
Current affiliation: Pyran
Age: 29
PhD alma mater: University of Wisconsin–Madison
Hometown: Cedar Hill, Texas
If I weren’t a chemist, I’d be: A geographer. “I could see myself traveling the world interacting with various peoples and their natural surroundings.”
If I were an element, I’d be: Copper, “because of its versatility (its ability to catalyze a range of reaction types that most other elements cannot) and its ability to work synergistically with others (its ability to readily combine with other elements to catalyze reactions at a faster rate than it could by itself).”
Just because something is possible doesn’t mean it is practical. That’s a mantra that William Banholzer’s students know all too well. Banholzer, a former chief technology officer of Dow Chemical Company, teaches a class at the University of Wisconsin–Madison, where he trains his students to evaluate the economic successes and failures of chemical companies.
Vitals
▸ Current affiliation: Pyran
▸ Age: 29
▸ PhD alma mater: University of Wisconsin–Madison
▸ Hometown: Cedar Hill, Texas
▸ If I weren’t a chemist, I’d be: A geographer. “I could see myself traveling the world interacting with various peoples and their natural surroundings.”
▸ If I were an element, I’d be: Copper, “because of its versatility (its ability to catalyze a range of reaction types that most other elements cannot) and its ability to work synergistically with others (its ability to readily combine with other elements to catalyze reactions at a faster rate than it could by itself).”
The course struck a chord with Kevin Barnett, who had recently joined the lab of renowned biofuels researcher George Huber. Barnett arrived at graduate school eager to combine his passion for the environment with his interest in chemical catalysis. But as Barnett soon learned, Banholzer is a vocal critic of the biofuels industry, where many scientifically ambitious ventures fail due to their economic infeasibilities.
So in Huber’s lab, Barnett began working on a Department of Energy–sponsored project to use renewable resources to make a high-value commodity chemical. The molecule, 1,5-pentanediol (1,5-PDO), is a building block of several polymers that are used to make a plethora of products including synthetic leather, high-performance paints, industrial coil coatings, adhesives, and more. Today, 1,5-PDO is made as a by-product from processing petroleum, and its relative rarity and high cost have limited its use.
In search of a cheaper, greener, and renewable source of the molecule, scientists have made 1,5-PDO from tetrahydrofurfuryl alcohol (THFA), a molecule derived from corncobs. Researchers discovered a catalytic reaction that creates 1,5-PDO in a single step by breaking the five-membered ring of THFA. “It was amazing chemistry,” Barnett says, but the catalysts it required were too expensive for wide adoption.
In Huber’s lab, Barnett sought a cheaper route to 1,5-PDO by dividing the single reaction into two simpler steps. “But no matter what I did, it wasn’t economical,” he says.
So he tried three steps.
“You might think that would be more costly,” he adds, but his elongated synthesis proved more efficient and selective at every step. Importantly, the penultimate step created an intermediate molecule with a ring that opened on its own—no costly catalyst needed.
Barnett spent the next 3 years optimizing the reaction. After graduating in 2018, he became the first employee, and CEO, of Pyran, a start-up that he and Huber cofounded to scale up and commercialize biobased 1,5-PDO. In the 2 years since, Barnett has conducted a market analysis, identified potential customers, pitched investors, hired the firm’s first two engineers, and scrutinized the spider web of products that currently use, or could use, 1,5-PDO. “Kevin did all the heavy lifting,” Huber says.
This year, Pyran plans to deliver its first 10–20 metric tons of product to its customers, priced at about $3,000 a metric ton—40% less than petroleum-derived 1,5-PDO sold by large chemical companies like BASF. Barnett estimates that the market for the molecule will be about $100 million–$200 million in a few years, although that figure could rise further in the future. Pyran is working with academic collaborators to explore making new polymers for 1,5-PDO, including biodegradable plastics—an application previously left untapped due to the molecule’s historically high cost.
While the success of biobased 1,5-PDO is no guarantee, Barnett’s pragmatism may help him navigate the start-up through a field littered with the remains of biobased chemical ventures whose economic compasses weren’t as finely tuned. And his practicality has even won over biobased fuel skeptic Banholzer, who joined Pyran’s board.
“It is a completely different animal,” Barnett says of his transition from laboratory scientist to budding chemical businessman. “But now that I am an entrepreneur, I don’t think there is ever any going back.”


















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter