Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Water
Jessica Ray
Water protector is developing ways to remove PFAS and other contaminants from this precious resource
by Katherine Bourzac
August 14, 2020
| A version of this story appeared in
Volume 98, Issue 31

Credit: University Of Washington (Ray); Shutterstock (coffee beans, water)
When Jessica Ray started her postdoctoral studies at the University of California, Berkeley, it was 2015, and the state was in the fourth year of what would be a 7-year drought. Droughts are nothing new in the western US, but there’s evidence they will become more intense and more frequent in the region, and many places around the world, because of climate change.
Ray’s time in dry California helped her focus in on her scientific mission. She wants to use her somewhat esoteric expertise in surface chemistry, materials science, and biogeochemistry to engineer more effective ways to treat water.
Advertisement
Ray finished undergrad with a degree in chemical engineering, but as she continued her studies, she was drawn to basic scientific research. Now she’s bringing them together, working as an environmental engineer whose practical solutions are built on some serious chemistry. “I want to make reactive materials and processes more useful for treating real water in the real world,” she says.
Particularly in arid coastal regions, Ray says, we need to get as much value as possible out of the precious water we have—contaminated though it may be. “Stormwater runoff is often viewed as a nuisance,” Ray says, but in fact it is a resource. One of her projects—currently on hold due to the pandemic—is aimed at turning a very Seattle sort of waste, used coffee grounds from the University of Washington, into biochar for rain gardens that can treat stormwater runoff. A priority is to keep chemicals that come from car tires out of the region’s creeks, where they can interrupt salmon spawning.
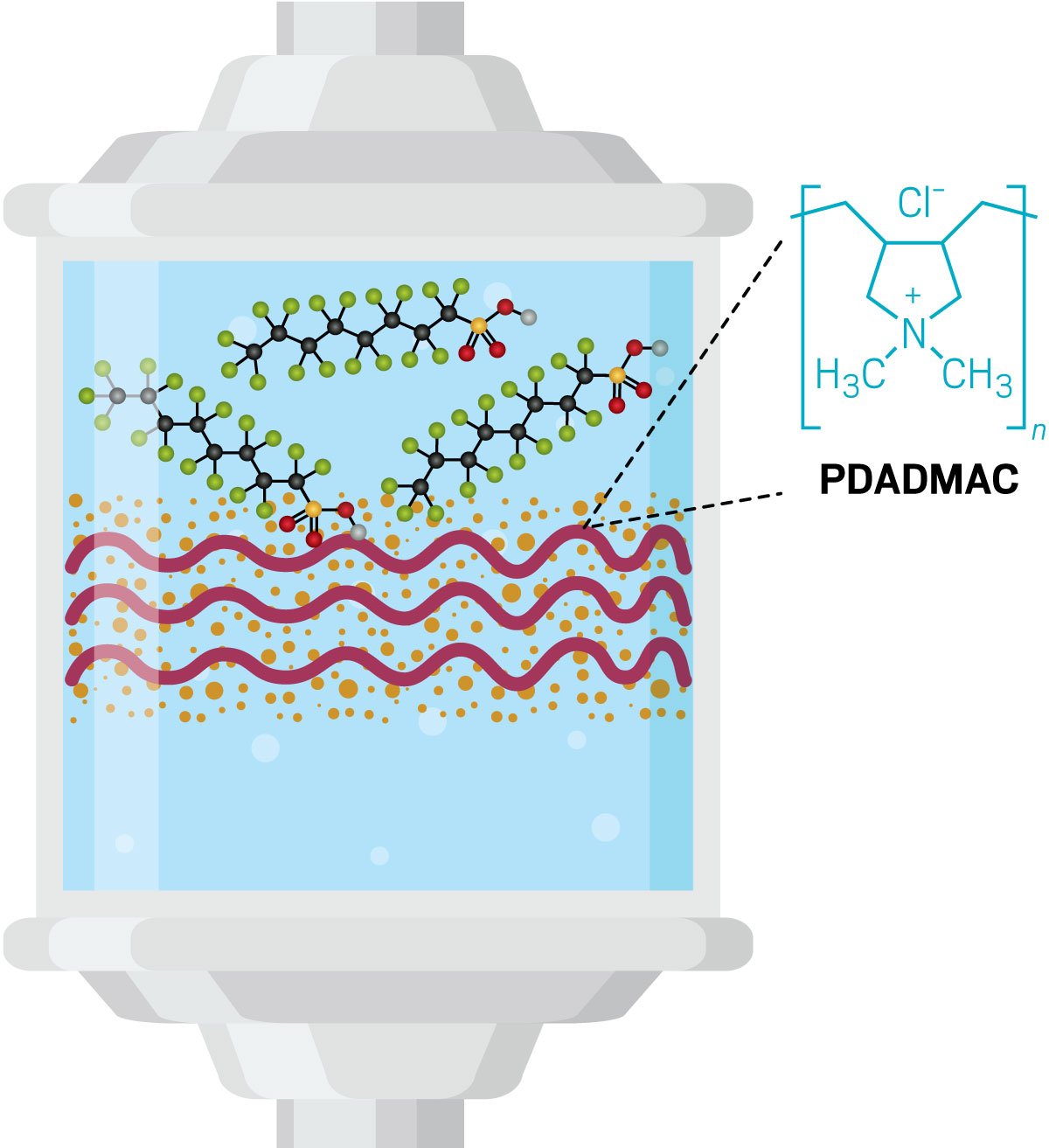
She’s also turning her expertise to one of the biggest problems in environmental chemistry: how to get rid of persistent organic pollutants called per- and polyfluoroalkyl substances (PFAS). “PFAS can become lost in large-scale water treatment,” Ray says. Granular activated carbon, the most common PFAS purifier, preferentially sops up more abundant organic contaminants, leaving behind trace, but still potentially harmful, levels of PFAS. Ray wants to “close the book on practical ways to manage PFAS,” she says.
During her postdoc, Ray developed a sand-like composite coated with polymers that target the carbon-fluorine PFAS backbone. The PFAS can then be selectively desorbed from the treatment medium, allowing Ray’s team to concentrate the pollutant in order to more effectively break it down.
The usual PFAS abatement methods require stringent conditions, such as the absence of oxygen or high pH. Since starting her lab last year, Ray is working on a simpler catalytic method for breaking down concentrated PFAS. It’s based on a relatively young class of 2-D electronic materials called MXenes, which have mostly been explored for use in energy storage. Their fast-charge kinetics and high surface area also make them promising electrocatalysts for oxidizing PFAS, says Ray. She’s eager to get back to the lab to test this PFAS filtration and destruction technology from start to finish using large filter columns and actual wastewater.
The planet’s water supplies are strained by pollution and climate change. But with some chemistry smarts, says Ray, environmental engineering can develop “healing applications.”
Vitals
Current affiliation: University of Washington
Age: 33
PhD alma mater: Washington University in St. Louis
Hometown: St. Louis, Missouri
If I weren’t a chemist, I’d be: A pastry chef. “I have a huge sweet tooth, so I started baking in graduate school to support my habit.”
If I were an element, I’d be: Sodium. “I love salty foods, especially sweet and salty foods, and firmly believe that sodium (in salt) makes everything taste better.”
When Jessica Ray started her postdoctoral studies at the University of California, Berkeley, it was 2015, and the state was in the fourth year of what would be a 7-year drought. Droughts are nothing new in the western US, but there’s evidence they will become more intense and more frequent in the region, and many places around the world, because of climate change.
Vitals
▸ Current affiliation: University of Washington
▸ Age: 33
▸ PhD alma mater: Washington University in St. Louis
▸ Hometown: St. Louis, Missouri
▸ If I weren’t a chemist, I’d be: A pastry chef. “I have a huge sweet tooth, so I started baking in graduate school to support my habit.”
▸ If I were an element, I’d be: Sodium. “I love salty foods, especially sweet and salty foods, and firmly believe that sodium (in salt) makes everything taste better.”
Ray’s time in dry California helped her focus in on her scientific mission. She wants to use her somewhat esoteric expertise in surface chemistry, materials science, and biogeochemistry to engineer more effective ways to treat water.
Ray finished undergrad with a degree in chemical engineering, but as she continued her studies, she was drawn to basic scientific research. Now she’s bringing them together, working as an environmental engineer whose practical solutions are built on some serious chemistry. “I want to make reactive materials and processes more useful for treating real water in the real world,” she says.
Particularly in arid coastal regions, Ray says, we need to get as much value as possible out of the precious water we have—contaminated though it may be. “Stormwater runoff is often viewed as a nuisance,” Ray says, but in fact it is a resource. One of her projects—currently on hold due to the pandemic—is aimed at turning a very Seattle sort of waste, used coffee grounds from the University of Washington, into biochar for rain gardens that can treat stormwater runoff. A priority is to keep chemicals that come from car tires out of the region’s creeks, where they can interrupt salmon spawning.
She’s also turning her expertise to one of the biggest problems in environmental chemistry: how to get rid of persistent organic pollutants called per- and polyfluoroalkyl substances (PFAS). “PFAS can become lost in large-scale water treatment,” Ray says. Granular activated carbon, the most common PFAS purifier, preferentially sops up more abundant organic contaminants, leaving behind trace, but still potentially harmful, levels of PFAS. Ray wants to “close the book on practical ways to manage PFAS,” she says.
During her postdoc, Ray developed a sand-like composite coated with polymers that target the carbon-fluorine PFAS backbone. The PFAS can then be selectively desorbed from the treatment medium, allowing Ray’s team to concentrate the pollutant in order to more effectively break it down.
The usual PFAS abatement methods require stringent conditions, such as the absence of oxygen or high pH. Since starting her lab last year, Ray is working on a simpler catalytic method for breaking down concentrated PFAS. It’s based on a relatively young class of 2-D electronic materials called MXenes, which have mostly been explored for use in energy storage. Their fast-charge kinetics and high surface area also make them promising electrocatalysts for oxidizing PFAS, says Ray. She’s eager to get back to the lab to test this PFAS filtration and destruction technology from start to finish using large filter columns and actual wastewater.
The planet’s water supplies are strained by pollution and climate change. But with some chemistry smarts, says Ray, environmental engineering can develop “healing applications.”




















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter