Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Biochemistry
Weixue Wang
This assay inventor identifies drug candidates for targets once thought to be undruggable
by Mark Peplow, special to C&EN
July 15, 2022
| A version of this story appeared in
Volume 100, Issue 25

Credit: Will Ludwig/C&EN/Tim Peacock/Scorpion Therapeutics
There's a thread running through Weixue Wang's scientific life that goes all the way back to his childhood in northern China. His father, who is a mechanic, ignited his son's enthusiasm for both science and engineering by encouraging him "to understand how things work and how things are built," Wang says. "That's always been my interest."
Pursuing that fascination with form and function at the molecular scale has led Wang to study how drug candidates interact with enzymes. As the director of biochemistry and biophysics at Scorpion Therapeutics, his work includes hunting for cancer-fighting compounds that target enzymes once thought to be undruggable. "It's a meaningful job because you can really help patients," he says.
Advertisement
Wang began working in the pharmaceutical industry in 2015, when he joined Janssen to develop biochemical and biophysical assays, which are fundamental tools in early-stage drug discovery. These tests screen libraries of compounds to find any that affect the activity of biological targets, such as enzymes, that may be involved in a disease. "He was the very first person I recruited in a group of 40, and he was just amazing," says Kay Ahn, who was Janssen's global head of molecular and cellular pharmacology at the time.
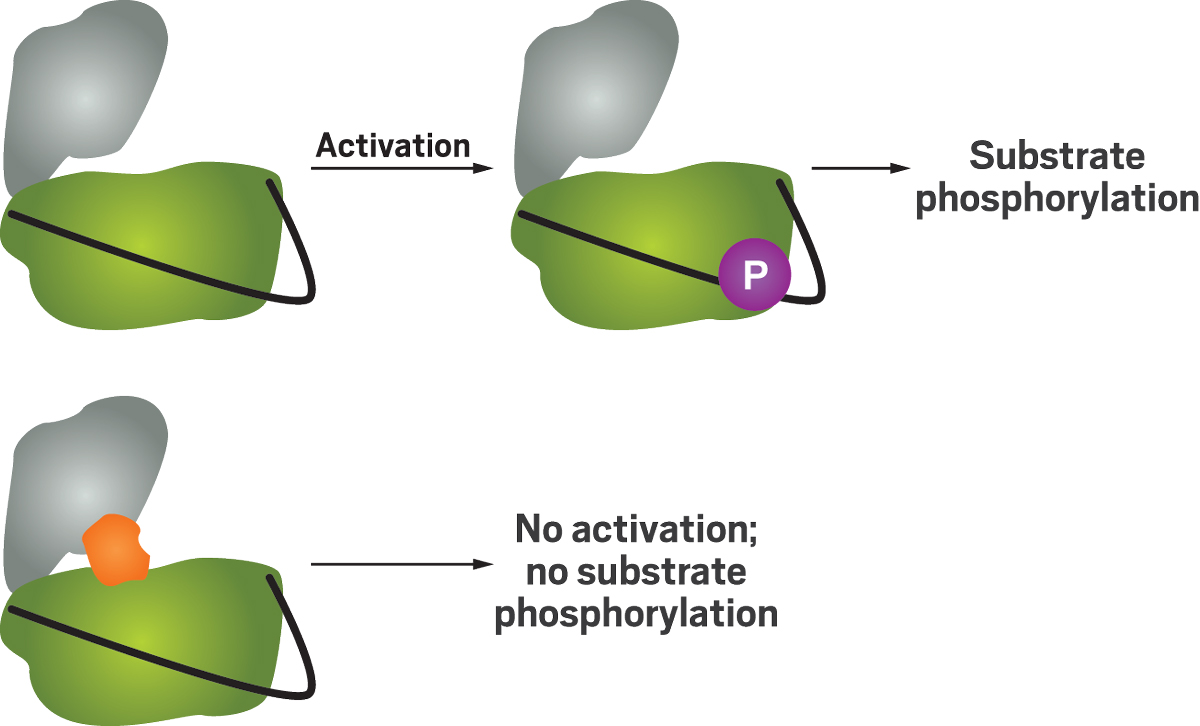
At Janssen, Wang developed a strategy to identify compounds that inhibit protein kinases. These enzymes can transfer a phosphate group from adenosine triphosphate to a protein. This phosphorylation process regulates protein function and cell signaling, and if it goes wrong, it sometimes causes cancer.
A traditional assay would look for compounds that bind to an activated kinase. But there are about 500 different protein kinases in humans, and when they are activated, their kinase domains—the business end of the enzyme—all have very similar structures. That similarity makes it difficult to find compounds that selectively hit the right kinase, and hitting the wrong one could lead to side effects.
Inactive kinases show a lot more structural variety, so compounds that target this form could be much more selective inhibitors. To snag those compounds, the researchers needed a new kind of assay.
Wang developed a method that involves adding compounds to the inactive kinase to discover whether they can bind to it and prevent the enzyme from switching to its active form. "From this type of assay we can find inhibitors that preferentially inhibit the inactive state," Wang says.
Wang identified the assay conditions by building a mathematical model of the complex reaction kinetics involved. "These conditions are very difficult to come up with just by intuition," Ahn says. His approach has been applied to a wide range of other kinases at Janssen and beyond, she adds.
In 2021, Wang brought his assay-wrangling skills to Scorpion Therapeutics, where he has helped identify an inhibitor of a mutant form of a phosphoinositide 3-kinase. Because the inhibitor selectively targets that mutant form, which is implicated in breast cancer, the compound will act only on the tumor, says Brent Martin, head of chemical biology at Scorpion Therapeutics. This selectivity also means the molecule could potentially avoid any toxicity from hitting kinases not associated with the cancer.
Wang's assays spotted the compound, called STX-478, and found that its selectivity for the mutant kinase was 10 times that for the normal kinase. Wang's kinetic modeling played a crucial role in designing assays to find the molecule, which is now being tested in animals. "Within literally 9 months, we went from a library hit to an in vivo active drug candidate," Martin says. "And that was totally driven by coming up with the right assays."
Vitals
Current affiliation: Scorpion Therapeutics
Age: 37
PhD alma mater: University of Illinois Urbana-Champaign
Hometown: Taiyuan, China
If I were an element, I'd be: “Carbon. Not highly reactive but is essential and versatile.”
My alternate-universe career: “There is a small possibility of me being an architect or archaeologist.”
See the Talented 12 present their work at a virtual symposium Sept. 19, 20, and 21. Register for free at cenm.ag/t12symposium.
|
|
There’s a thread running through Weixue Wang’s scientific life that goes all the way back to his childhood in northern China. His father, who is a mechanic, ignited his son’s enthusiasm for both science and engineering by encouraging him “to understand how things work and how things are built,” Wang says. “That’s always been my interest.”
Vitals
▸ Current affiliation: Scorpion Therapeutics
▸ Age: 37
▸ PhD alma mater: University of Illinois Urbana-Champaign
▸ Hometown: Taiyuan, China
▸ If I were an element, I’d be: “Carbon. Not highly reactive but is essential and versatile.”
▸ My alternate-universe career: “There is a small possibility of me being an architect or archaeologist.”
Pursuing that fascination with form and function at the molecular scale has led Wang to study how drug candidates interact with enzymes. As the director of biochemistry and biophysics at Scorpion Therapeutics, his work includes hunting for cancer-fighting compounds that target enzymes once thought to be undruggable. “It’s a meaningful job because you can really help patients,” he says.
Wang began working in the pharmaceutical industry in 2015, when he joined Janssen to develop biochemical and biophysical assays, which are fundamental tools in early-stage drug discovery. These tests screen libraries of compounds to find any that affect the activity of biological targets, such as enzymes, that may be involved in a disease. “He was the very first person I recruited in a group of 40, and he was just amazing,” says Kay Ahn, who was Janssen’s global head of molecular and cellular pharmacology at the time.
At Janssen, Wang developed a strategy to identify compounds that inhibit protein kinases. These enzymes can transfer a phosphate group from adenosine triphosphate to a protein. This phosphorylation process regulates protein function and cell signaling, and if it goes wrong, it sometimes causes cancer.
A traditional assay would look for compounds that bind to an activated kinase. But there are about 500 different protein kinases in humans, and when they are activated, their kinase domains—the business end of the enzyme—all have very similar structures. That similarity makes it difficult to find compounds that selectively hit the right kinase, and hitting the wrong one could lead to side effects.
Inactive kinases show a lot more structural variety, so compounds that target this form could be much more selective inhibitors. To snag those compounds, the researchers needed a new kind of assay.
Wang developed a method that involves adding compounds to the inactive kinase to discover whether they can bind to it and prevent the enzyme from switching to its active form. “From this type of assay we can find inhibitors that preferentially inhibit the inactive state,” Wang says.
Wang identified the assay conditions by building a mathematical model of the complex reaction kinetics involved. “These conditions are very difficult to come up with just by intuition,” Ahn says. His approach has been applied to a wide range of other kinases at Janssen and beyond, she adds.
In 2021, Wang brought his assay-wrangling skills to Scorpion Therapeutics, where he has helped identify an inhibitor of a mutant form of a phosphoinositide 3-kinase. Because the inhibitor selectively targets that mutant form, which is implicated in breast cancer, the compound will act only on the tumor, says Brent Martin, head of chemical biology at Scorpion Therapeutics. This selectivity also means the molecule could potentially avoid any toxicity from hitting kinases not associated with the cancer.
Wang’s assays spotted the compound, called STX-478, and found that its selectivity for the mutant kinase was 10 times that for the normal kinase. Wang’s kinetic modeling played a crucial role in designing assays to find the molecule, which is now being tested in animals. “Within literally 9 months, we went from a library hit to an in vivo active drug candidate,” Martin says. “And that was totally driven by coming up with the right assays.”


















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter