Prevent waste

Running reactions in one pot can reduce waste from workups between each step.
Credit: Shutterstock
“It is better to prevent waste than to treat or clean up waste after it has been created.”
The preventive principle is central to green chemistry. One powerful way this idea plays out is through one-pot synthesis. Instead of running a multistep reaction in numerous flasks with solvent and energy-intensive workups between each step, scientists can do one-pot reactions, which are designed to take place in a single flask without researchers’ needing to stop and purify. One recent example is the biotechnology company Amgen’s synthesis of the lung cancer drug Lumakras (sotorasib). The traditional method for making the drug creates two versions with different potencies. Amgen’s scientists cut several isolation and purification steps to create a one-pot reaction that converts the unwanted version of the drug into the more potent form. Amgen estimated that the new process will generate about 14.4 million kg less waste per year. Redesigns to decrease the number of synthetic steps can also greatly reduce waste. In 2020, Merck & Co. reported a more efficient process to make gefapixant citrate, a drug treating chronic coughs (Org. Process Res. Dev. 2020, DOI: 10.1021/acs.oprd.0c00248). Merck scientists reduced the number of steps from 10 to 2, which also cut the amount and cost of materials needed to make the drug, and increased yield by 44%.
Economize atoms
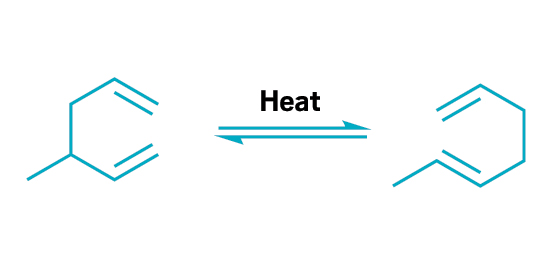
The Cope rearrangement is the reversible reaction of 1,5-dienes and related compounds to form new dienes with high atom economy.
“Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.”
This principle embodies atom economy—the number of atoms in a chemical reaction that are turned into product. To calculate atom economy, chemists divide the molecular weight of a reaction’s final product by the total molecular weight of all reactants. This calculation is in contrast to percent yield, the ratio of the actual product yield to the theoretical product yield of a reaction. Instead of assessing just the amount of product, as with percent yield, atom economy considers all the reactants created, including waste products. Abiding by this principle minimizes waste and optimizes the use of resources. Many well-known synthetic reactions have high atom economy, especially when chemists break the bonds of one molecule to form a new compound with the same atoms. Examples include olefin metathesis, coupling reactions such as the Suzuki-Miyaura cross coupling, cyclization reactions such as the Diels-Alder reactions, and rearrangements such as Claisen and Cope reactions.
Make it benign

US Environmental Protection Agency researchers are developing tools such as Generalized Read-Across (GenRA) to rapidly test chemicals for effects such as endocrine disruption.
Credit: EPA
“Chemical products should be designed to affect their desired function while minimizing their toxicity.”
This principle is also called the design of benign chemicals. Scientists have developed many methods and resources that can help chemists choose and synthesize compounds that are less likely to be environmental or health hazards. The US Environmental Protection Agency developed an algorithm called Generalized Read-Across (GenRA) that predicts the in vivo toxicity and in vitro bioactivity of uncharacterized compounds by looking for similarities with known compounds’ structures or chemical activity. By using this tool, researchers can help determine chemicals’ harmful properties without having to test each one. Another EPA tool, a toxicity forecaster called ToxCast, uses high-throughput, cell-based assays and computational methods to predict the toxicity of thousands of chemicals. The method generates data and models of how the compounds may affect humans and animals in the environment. Scientists can download ToxCast data and access GenRA from the EPA’s website or use GenRA with the CompTox Chemicals Dashboard.
The structure-activity relationship (SAR) and quantitative structure-activity relationship (QSAR) are methods used in drug discovery that can help minimize new compounds’ toxicity. Both approaches connect a molecule’s biological activity with its 3D structure and can predict toxicity and function.
Ditch harmful solvents

Organic-solvent waste can harm people’s health and the environment, so some scientists are trying to reduce use.
Credit: Shutterstock
“The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and innocuous when used.”
Solvents have long been a mainstay for running reactions, but solventless processes such as mechanochemistry are gaining traction. Ball milling, in which chemists load solid reactants into a vessel with steel balls and then shake or rotate the reaction at high speeds, is a type of mechanochemistry. Separate teams of researchers have reported running Haber-Bosch reactions using ball mills instead of the traditional industrial method to make ammonia, which causes roughly 1.3% of human-made carbon dioxide emissions per year, according to the International Energy Agency.
Switching from organic solvents to water is another way to reduce hazardous solvent waste. Chemists have developed several methods to trick organic molecules into reacting in water, such as using surfactants or biocatalysis or doing reactions at the water-oil interface. For example, the Suzuki-Miyaura cross-coupling reaction, widely used in organic synthesis, can be performed in water along with a palladium catalyst and the micelle-forming compound polyoxyethanyl α-tocopheryl sebacate (Org. Lett. 2008, DOI: 10.1021/ol801712e). The method not only eliminates harmful solvents but requires heating to only 38 °C instead of over 70 °C and thus uses less energy than the traditional Suzuki-Miyaura reaction.
Power with catalysts
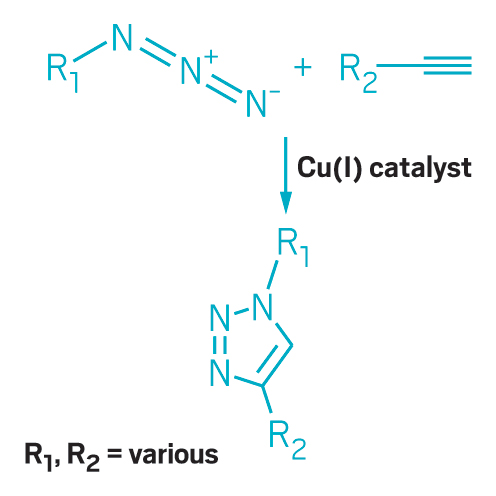
The copper-catalyzed azide-alkyne cycloaddition is click chemistry's premier reaction.
“Catalytic reagents (as selective as possible) are superior to stoichiometric reagents.”
Catalysts are crucial to green chemistry because they enable reactions to do more with less. Instead of using reactants in stoichiometric ratios, researchers can use catalysts, which react with multiple molecules of starting material, eliminating the need for large amounts of reactants and thus reducing the amount of waste produced. Several standout reactions get their efficiency from catalytic reagents. Olefin metathesis, in which carbon-carbon double bonds break and re-form with new partners, garnered Yves Chauvin, Robert H. Grubbs, and Richard R. Schrock a Nobel Prize in Chemistry in 2005. The metal-carbene catalysts, commonly known as Grubbs catalysts, are widely used in organic synthesis.
Copper-catalyzed azide-alkyne cycloaddition, also known as click chemistry, offers another way to reduce chemical waste. Carolyn R. Bertozzi, Morten Meldal, and K. Barry Sharpless were awarded the 2022 Nobel Prize in Chemistry for discovering it and bioorthogonal chemistry. The method allows chemists to add functional groups to compounds later in a synthesis than previous approaches. With this ability, researchers avoid installing and later removing protecting groups that shield sensitive parts of the molecule from being altered by unwanted reactions. The standard protection process is laborious and generates a lot of waste solvent. Click chemistry has found broad applications in drug discovery, materials science, and bioconjugation.
Design to degrade

Encapsulated enzymes distributed throughout polycaprolactone plastic film can accelerate the breakdown of the material in the presence of heat and humidity.
Credit: Nature
“Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment.”
Until relatively recently, industry generally didn’t favor chemical compounds that break down quickly. But a growing awareness of the negative effects of persistent organic pollutants, per- and polyfluoroalkyl substances, and plastic pollution is driving chemists to focus on more degradable compounds and materials to minimize potentially harmful environmental impacts and promote sustainability.
One example is the development of biodegradable polymers to replace conventional plastics such as polyethylene. To make this polymer break apart under sunlight after a few months, chemists added ketones to its long hydrocarbon chains (Science 2021, DOI: 10.1126/science.abi8183). Polycaprolactone (PCL) and polylactic acid (PLA) are also biodegradable plastics and are used for food containers and dog-poop bags. These polymers need high temperatures to break down, so researchers incorporated nanoparticles with plastic-chomping enzymes into their design (Nature 2021, DOI: 10.1038/s41586-021-03408-3). Humidity and temperatures of 40–60 °C unleashed the enzymes, which chewed the polymers to bits in hours to days.















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter