Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Alexandra Velian
This inorganic chemist builds catalytic clusters that perform new chemical tricks
by Leigh Krietsch Boerner
July 15, 2022
| A version of this story appeared in
Volume 100, Issue 25

Credit: Will Ludwig/C&EN/Tim Peacock/Dennis R. Wise
Alexandra Velian took her first-ever airplane flight when she moved from her native Romania to Los Angeles to go to the California Institute of Technology for college. In high school, she knew she wanted to study chemistry, but she quickly realized that to get on the scientific path she wanted, she’d have to travel far to a place she knew little about.
Velian has always been willing to take the plunge into the unknown. Today, in her research at the University of Washington, she’s trying to push inorganic materials to learn tricks previously thought impossible.
Advertisement
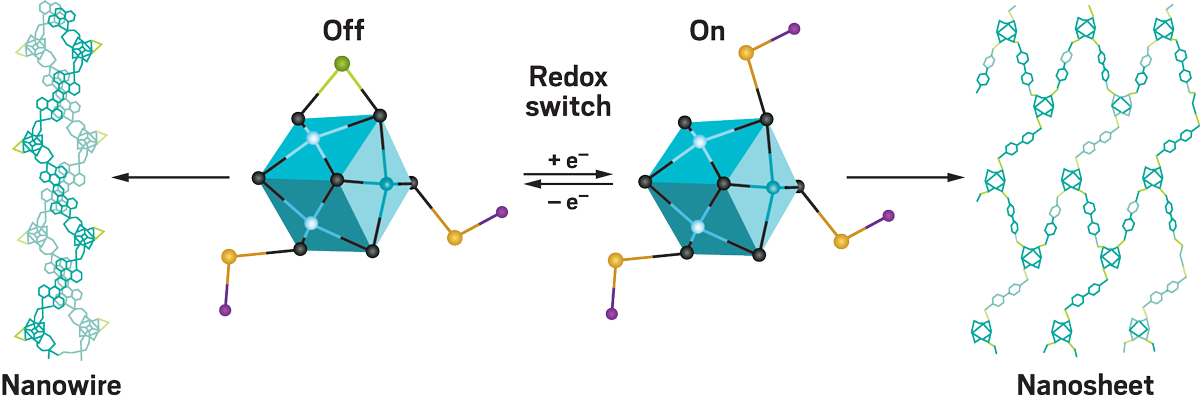
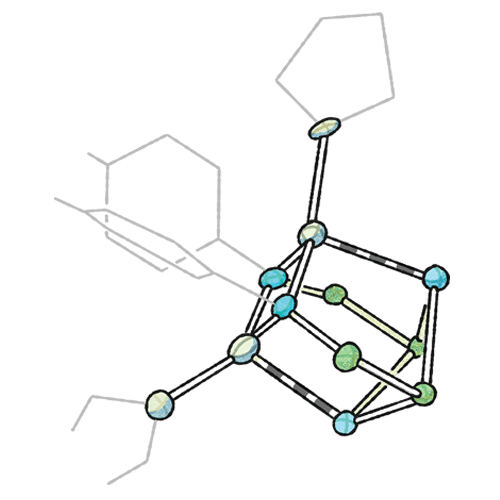
Velian works on materials made from inorganic clusters of atoms, which she and her lab design with very precise functions. In particular, they construct catalytic clusters that can respond to molecules in their environments. Inorganic chemists aren’t accustomed to this level of control. “In organic chemistry, we’re pretty good at controlling carbon-carbon bonds and making very sophisticated molecules that perform specific functions in the body,” Velian says. “In inorganic chemistry, that’s a bit harder to do.”
By developing such control, Velian hopes to create inorganic materials that can, for example, selectively react with a specific pathway of C–C bond formation in carbon dioxide reduction. Another possibility is that the catalysts could change their chemistry after one active site on a catalyst binds a specific type of molecule.
Although Velian’s clusters can act as catalysts themselves, her lab also wants to find ways to make the clusters assemble into bigger materials. Her lab takes clusters it has designed with special abilities and sticks them together like Lego blocks, or it starts with existing materials and builds catalytic sites into them. “Once these clusters are linked together in a larger assembly, you can get something that’s greater than the sum of its parts,” Velian says. So far, Velian has led the way in determining these clusters’ detailed structures and is using this knowledge to understand how the clusters interact to form materials.
“In a very short period of time, she has carved out a space and put forward a new approach,” says Brandi Cossairt, who is an inorganic chemist at the University of Washington and was a graduate school lab mate of Velian’s at the Massachusetts Institute of Technology. “She just aspires to greatness and brings that out in her students. I find her very inspiring,” Cossairt says.
Velian enjoys pushing into the unknown, but she admits it does pose some challenges. She works in an emerging area of research, so there’s not a lot of established science to build on. Plus, scientists working on cluster-assembled materials come from multiple disciplines, not just chemistry. “We have to learn to speak physics and speak surface science, when we are at heart synthetic inorganic chemists,” Velian says. Working at the interface of multiple fields leads to unique opportunities, she says, but the path definitely presents different challenges from going into research similar to what your mentors have done.
But veering off in her own direction has been a guiding force in Velian’s life. “Being at the frontier means I’m doing the chemistry that I’m most passionate about,” she says.
Vitals
Current affiliation: University of Washington
Age: 36
PhD alma mater: Massachusetts Institute of Technology
Hometown: Tulcea, Romania
If I were an element, I'd be: “Hydrogen because I would be at the heart of every star in the universe.”
My alternate-universe career: “A natural historian. I’d love to work with David Attenborough and his team to observe and report on the world around us.”
See the Talented 12 present their work at a virtual symposium Sept. 19, 20, and 21. Register for free at cenm.ag/t12symposium.

|
|
Alexandra Velian took her first-ever airplane flight when she moved from her native Romania to Los Angeles to go to the California Institute of Technology for college. In high school, she knew she wanted to study chemistry, but she quickly realized that to get on the scientific path she wanted, she’d have to travel far to a place she knew little about.
Vitals
▸ Current affiliation: University of Washington
▸ Age: 36
▸ PhD alma mater: Massachusetts Institute of Technology
▸ Hometown: Tulcea, Romania
▸ If I were an element, I’d be: “Hydrogen because I would be at the heart of every star in the universe.”
▸ My alternate-universe career: “A natural historian. I’d love to work with David Attenborough and his team to observe and report on the world around us.”
Velian has always been willing to take the plunge into the unknown. Today, in her research at the University of Washington, she’s trying to push inorganic materials to learn tricks previously thought impossible.
Velian works on materials made from inorganic clusters of atoms, which she and her lab design with very precise functions. In particular, they construct catalytic clusters that can respond to molecules in their environments. Inorganic chemists aren’t accustomed to this level of control. “In organic chemistry, we’re pretty good at controlling carbon-carbon bonds and making very sophisticated molecules that perform specific functions in the body,” Velian says. “In inorganic chemistry, that’s a bit harder to do.”
By developing such control, Velian hopes to create inorganic materials that can, for example, selectively react with a specific pathway of C-C bond formation in carbon dioxide reduction. Another possibility is that the catalysts could change their chemistry after one active site on a catalyst binds a specific type of molecule.
Although Velian’s clusters can act as catalysts themselves, her lab also wants to find ways to make the clusters assemble into bigger materials. Her lab takes clusters it has designed with special abilities and sticks them together like Lego blocks, or it starts with existing materials and builds catalytic sites into them. “Once these clusters are linked together in a larger assembly, you can get something that’s greater than the sum of its parts,” Velian says. So far, Velian has led the way in determining these clusters’ detailed structures and is using this knowledge to understand how the clusters interact to form materials.
“In a very short period of time, she has carved out a space and put forward a new approach,” says Brandi Cossairt, who is an inorganic chemist at the University of Washington and was a graduate school lab mate of Velian’s at the Massachusetts Institute of Technology. “She just aspires to greatness and brings that out in her students. I find her very inspiring,” Cossairt says.
Velian enjoys pushing into the unknown, but she admits it does pose some challenges. She works in an emerging area of research, so there’s not a lot of established science to build on. Plus, scientists working on cluster-assembled materials come from multiple disciplines, not just chemistry. “We have to learn to speak physics and speak surface science, when we are at heart synthetic inorganic chemists,” Velian says. Working at the interface of multiple fields leads to unique opportunities, she says, but the path definitely presents different challenges from going into research similar to what your mentors have done.
But veering off in her own direction has been a guiding force in Velian’s life. “Being at the frontier means I’m doing the chemistry that I’m most passionate about,” she says.


















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter