RNA
This was mRNA’s breakout year, and scientists are just getting started
Start-ups are securing money for new applications of mRNA and new versions of the technology
by Ryan Cross
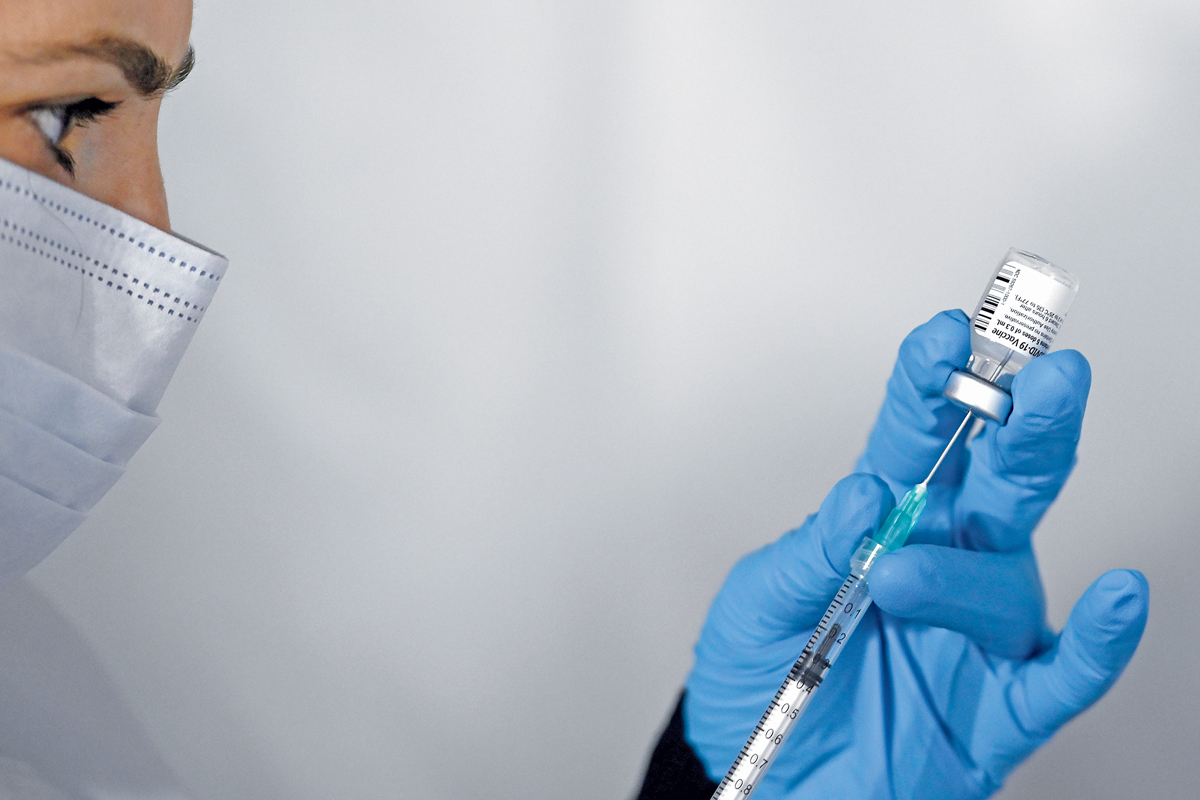
It’s easy to forget that just 2 years ago most people had never heard of messenger RNA (mRNA) vaccines. Among those who had, many were skeptical that the technology would work. Even if mRNA vaccines proved to be safe and effective, manufacturing the genetic molecules in vast amounts seemed like a distant goal. COVID-19 changed all of that virtually overnight.
Before 2020, the number of people to get an experimental injection of an mRNA therapy or vaccine numbered only in the low thousands. This year, BioNTech, Pfizer, and Moderna distributed billions of their mRNA-based vaccines around the world. By late November, medical professionals had administered more than 437 million shots of an mRNA vaccine in the US alone.
No one expected the first commercial mRNA products to touch so many lives—or make their developers so much money. Moderna estimates it will sell up to $18 billion of its vaccine this year, and Pfizer pins its estimate twice as high, $36 billion. The drug industry no longer doubts mRNA’s worth. The question is now: What can mRNA do next?
For scientists working with the molecule, the applications seem nearly endless. mRNA can encode instructions that teach our cells how to make any protein. Companies are developing mRNA vaccines for influenza, respiratory syncytial virus, malaria, and more. Other firms are using mRNA to encode antibodies, cancer immunotherapies, protein replacement therapies, and CRISPR gene-editing systems. mRNA, it seems, can do it all.
“It is a totally different way of thinking about drugs,” says Diego Miralles, CEO of the RNA start-up Laronde, which raised $440 million in August. “We are entering a new era of medicine.”
Although scientists were studying many of mRNA’s potential applications before the pandemic, the companies working on them are getting more attention—and investment—thanks to the triumph of the COVID-19 vaccines. “Nothing encourages investment like success,” says Thomas Barnes, CEO of the RNA start-up Orna Therapeutics, which raised $80 million in February.
Laronde and Orna are just two of the biotech firms that emerged from stealth this year with big piles of cash to develop new versions of mRNA technology. Both start-ups are making therapies based on circular RNA molecules, which they believe will be safer and more durable than linear mRNA.
Two other start-ups, Kernal Biologics and Strand Therapeutics, are programming mRNA-based cancer therapies that activate only in certain cells. The approach could minimize side effects.
And Replicate Bioscience and VaxEquity are developing what they call self-replicating or self-amplifying RNA technology, which uses viral genes to help the RNA molecules multiply inside the body. The approach could allow the products to be administered at lower doses than traditional mRNA. “It has just been a huge amount of momentum that the field has gotten this year,” says Anna Blakney, an RNA scientist at the University of British Columbia, Vancouver, and a cofounder of VaxEquity.
The know-how and infrastructure set up to produce the mRNA COVID-19 vaccines will help second-generation versions of the technology move faster, says Nathaniel Wang, CEO and cofounder of Replicate Bioscience. “We don’t have to invent how to make mRNA,” he says. Contract manufacturing firms already solved that problem during the pandemic. That means “we can go fast. That’s why there is a lot of money coming in,” he says. “I think there is plenty of room for a lot of different winners to emerge.”
Although the effectiveness of the mRNA COVID-19 vaccines is clear, scientists debate how much of that success is due to mRNA technology itself. Although the mRNA vaccines were more effective than the adenoviral vector vaccines made by AstraZeneca and Johnson & Johnson, a protein-based vaccine from Novavax seemed about as effective as the mRNA vaccines, explains Andrew Geall, Replicate’s cofounder and chief development officer.
But slower-moving clinical trials and manufacturing delays have kept Novavax’s shot from reaching many people thus far. “The only advantage of RNA at the moment is speed,” Geall says.
Making a good mRNA vaccine requires knowing what antigen to encode in it. For the COVID-19 vaccines, the antigen was the spike protein from the SARS-CoV-2 virus. Years of work on similar proteins from other coronaviruses helped scientists design vaccines for COVID-19 quickly.
The pandemic showed that “if you have a good antigen, you can rely on mRNA to express it effectively and stimulate robust immune responses,” says Yusuf Erkul, CEO and cofounder of Kernal Biologics. “But that doesn’t mean it can’t be improved.”
Creating shots with fewer and less-intense side effects will likely be key for companies wanting to develop mRNA vaccines for common infections like the flu, Blakney says.
“I can’t imagine that many people would want to get a flu vaccine every year that has the same side effects as the COVID vaccine,” she says. “The day after I got my Moderna booster, I literally couldn’t move from the couch.” Blakney thinks the key to reducing these side effects lies in finding a way to dramatically decrease the dose of the shots without sacrificing their effectiveness.
One potential area for improvement is in the delivery of mRNA into the right cells, either with new lipid nanoparticles—how mRNA is currently delivered—or another delivery technology altogether. While vaccines can simply be jabbed into the arm, where they stimulate immune cells, new delivery strategies will be required for mRNA therapies that need to reach particular parts of the body, such as the brain.
Right now, targeted mRNA delivery is limited primarily to the liver, lungs, and spleen, says Kathryn Whitehead, a drug delivery scientist at Carnegie Mellon University. “Delivery is, and will continue to be, the most significant barrier to implementing mRNA therapeutics for a wide array of diseases,” she says.
The pandemic has spurred pharmaceutical firms to up their investment in mRNA. Pfizer, which partnered with BioNTech for its COVID-19 vaccine, is working on its own mRNA vaccines. Sanofi acquired its mRNA partner, Translate Bio, for $3.2 billion and said it would spend an additional $475 million annually on its newly launched mRNA Center of Excellence. And AstraZeneca struck a partnership with VaxEquity to develop therapies with the start-up’s self-amplifying RNA.
“The potential has been confirmed, and that is why we see so many start-ups and so much investment in this space,” says Michael Watson, director and executive chairman of VaxEquity.
But not every mRNA program has been successful. Translate Bio posted disappointing results in March from its early trial of an mRNA therapy for cystic fibrosis.
Sanofi also decided to terminate Translate Bio’s lagging COVID-19 vaccine program and shifted its research focus to mRNA vaccines for other diseases, including the flu. And CureVac’s mRNA vaccine for COVID-19 did not trigger as strong of an immune response as did the Moderna and Pfizer-BioNTech vaccines. The company stopped developing it and instead is focusing on a second-generation version.
Translate Bio and CureVac used a slightly different version of mRNA technology for their COVID-19 vaccines than Moderna and BioNTech. While the latter firms used chemically modified nucleotides designed to avoid triggering problematic immune responses, Translate Bio and CureVac used unmodified nucleotides.
But since the doses, lipid nanoparticles, and genetic sequences also varied among the four vaccines, some scientists say it is hard to know exactly why the Translate Bio and CureVac programs failed to impress. “It is just really hard to compare those clinical trials,” Blakney says.
mRNA companies have also been the subject of intense criticism for tightly controlling production of their vaccines and refusing to share patents and technical know-how with groups in low- and middle-income countries, where many people are still waiting for their first shots. Moderna and BioNTech both announced plans to build large mRNA vaccine manufacturing facilities in Africa, but the projects do not solve the immediate need for vaccines on the continent.
More recently, a feud between Moderna and the US National Institutes of Health over who owns Moderna’s COVID-19 vaccine has reached a boiling point. The NIH says its scientists helped design the vaccine and should share ownership of the core patents, while Moderna asserts that the vaccine that was ultimately approved for use in humans was a product of its own scientists’ design.
Despite the controversies, many scientists working on mRNA are excited about the increased attention and accelerated investment the field is experiencing. “It has become a lot more challenging to keep up with the speed of innovation in the mRNA field now,” Kernal’s Erkul says.
The potentially broad application of mRNA reminds people of the early days of protein-based therapies—which the industry calls biologics. For a long time, biologics were hard to develop, and leaders in the space were few, VaxEquity’s Watson says. “Today most pharma companies have a very significant, if not majority, activity in biologics, so I don’t see any reason why RNA can’t eventually become as significant a part of medicine.”







Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter