Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Drug Delivery
Covid-19
Without these lipid shells, there would be no mRNA vaccines for COVID-19
Fragile mRNA molecules used in COVID-19 vaccines can’t get into cells on their own. They owe their success to lipid nanoparticles that took decades to refine
by Ryan Cross
March 6, 2021
| A version of this story appeared in
Volume 99, Issue 8

Messenger RNA (mRNA) is having a moment. This year, hundreds of millions of people will receive shots of the Pfizer-BioNTech or Moderna vaccines for COVID-19. The crucial ingredient in each injection is mRNA, short-lived strands of genetic material that prompt our cells to start making SARS-CoV-2 proteins, which in turn help our immune systems develop antibodies that prevent future infections. Thanks to decades of scientific perseverance, billions of dollars of investment in the technology, and previous work on coronaviruses, the vaccine makers were able to design their vaccines and prove their safety and efficacy in under a year.
The success of these COVID-19 vaccines is remarkable and was far from guaranteed. mRNA is incredibly delicate. Enzymes in the environment and in our bodies are quick to chop mRNA into pieces, making lab experiments difficult and the delivery of mRNA to our cells daunting. On top of that, mRNA strands are large and negatively charged and can’t simply waltz across the protective lipid membranes of cells. Many scientists thought the technology would never work.
“There were many, many skeptics,” says Frank DeRosa, who began working with mRNA in 2008 and is now chief technology officer at Translate Bio, a firm developing mRNA vaccines with Sanofi. “People used to say that if you looked at it wrong it would fall apart.”
Luckily, scientists found a solution. To protect the fragile molecule as it sneaks into cells, they turned to a delivery technology with origins older than the idea of mRNA therapy itself: tiny balls of fat called lipid nanoparticles, or LNPs.
LNPs used in the COVID-19 vaccines contain just four ingredients: ionizable lipids whose positive charges bind to the negatively charged backbone of mRNA, pegylated lipids that help stabilize the particle, and phospholipids and cholesterol molecules that contribute to the particle’s structure. Thousands of these four components encapsulate mRNA, shield it from destructive enzymes, and shuttle it into cells, where the mRNA is unloaded and used to make proteins. Although the concept seems simple, perfecting it was far from straightforward.
Over more than 3 decades, promising lipids studied in the lab often failed to live up to their potential when tested in animals or humans. Positively charged lipids are inherently toxic, and companies struggled for years before landing on formulations that were safe and effective. When injected intravenously, the particles invariably accumulated in the liver, and delivery to other organs is still an obstacle. Reliably manufacturing consistent LNPs was another challenge, and producing the raw materials needed to make the particles is a limiting factor in the production of COVID-19 vaccines today.
LNP development has been a headache, but without this packaging, mRNA vaccines would be nothing. “It is the unsung hero of the whole thing,” says Giuseppe Ciaramella, who was head of infectious diseases at Moderna from 2014 to 2018.
The vaccines, appropriately celebrated as a first for mRNA technology, are also a milestone for the nanoparticle field. Although the first drug based on an LNP was approved by the US Food and Drug Administration for a rare genetic disease in 2018, the two authorized mRNA vaccines for COVID-19 present a far bigger opportunity for the nanoparticles than even the field’s founders can imagine. “It is a tremendous vindication for everyone working in controlled drug delivery,” says Robert Langer, a chemical engineer at the Massachusetts Institute of Technology.
“LNPs will be going into millions of arms over the course of this year,” says University of British Columbia nanoparticle scientist Pieter Cullis. “What was a fringe field back in the 1980s has turned into something that is mainstream now.”
The delivery dilemma
Modern LNPs can be traced back to work on simpler systems called liposomes, hollow lipid spheres often made of just two or three kinds of lipids. In the early 1980s, Cullis found that cancer drugs could diffuse into these liposomes and get trapped in the hollow core. When injected into animals with cancer, the liposomes would slip through the leaky vasculature of tumors, enter cells, and unleash a drug. Cullis, and several others, started companies with the hope that liposomes could safely deliver otherwise toxic drugs into tumors in humans.
Progress was slowed by issues with stability and manufacturing. The first liposome-based drug eventually was approved by the FDA in 1995, but by then Cullis and many in the field had moved on to a new challenge: using lipid particles to deliver nucleic acids such as DNA and RNA.
At the time, scientists were enamored by advances in genetics that were promising to cure diseases by giving someone new genes or turning disease-causing genes off. Figuring out how to deliver these nucleic acid therapies—either DNA or RNA—into cells was a major challenge and required something more sophisticated than a conventional liposome. Cullis knew that adding positively charged lipids to the liposomes would help balance the negatively charged nucleic acids, but there was a problem. “There are no cationic lipids in nature,” Cullis says. “And we knew we couldn’t use permanently positively charged lipids because they are so damn toxic.” Those lipids would rip cell membranes apart, he adds.
A solution came from new lipids that were charged only under certain conditions. During the late ’90s and through the first decade of the 2000s, Cullis, his colleagues at Inex Pharmaceuticals, and the Inex spin-off Protiva Biotherapeutics developed ionizable lipids that are positively charged at an acidic pH but neutral in the blood. The group also created a new way to manufacture nanoparticles with these lipids, using microfluidics to mix lipids dissolved in ethanol with nucleic acids dissolved in an acidic buffer. When the streams of those two solutions merged, the components spontaneously formed lipid nanoparticles, which, unlike the hollow liposomes, were densely packed with lipids and nucleic acids. The process was simple in theory, but getting the machine to reliably spit out consistent LNPs was difficult.
LNPs that looked good in the lab often floundered in the clinic, however. The first versions of ionizable lipids were still toxic. And early formulations of the nanoparticles didn’t degrade fast enough, causing them to accumulate after repeated injections. Protiva found that one of its experimental LNP therapies caused a more severe immune reaction in humans than it had in the lab, and the company pinned pegylated lipids as a major factor.
Pegylated lipids, in which polyethylene glycol (PEG) strands are attached to lipid heads, have several functions in a nanoparticle. PEG helps control the particle size during formulation, prevents the particles from aggregating in storage, and initially shields the particles from being detected by immune system proteins in the body, according to James Heyes, a former Protiva scientist. Heyes is now chief scientific officer of the LNP company Genevant Sciences—a firm with origins in Protiva.
But PEG also has liabilities. It prevents LNPs from binding to proteins that help shuttle them into cells. Because PEG extends particles’ life span in the body, the immune system has more time to spot the particles and start mounting an antibody response. And although PEG is found in many cosmetic, drug, and food products, scientists hypothesize that some people could develop antibodies to PEG and that giving those individuals an injection of PEG-coated nanoparticles could trigger an anaphylactic reaction.
Escape from the endosome
By 2005, the development of better and safer LNPs was driven by excitement for a new technology, called small interfering RNA (siRNA), for selectively silencing genes. Alnylam Pharmaceuticals, which became the leading siRNA company, quickly realized that existing nanoparticles were not very good at helping siRNA get into cells. The company struck multiple partnerships to make new LNPs, including with Protiva in 2005 and Inex in 2006. The groups made more than 300 ionizable lipids, first optimizing the fatty tails, then tweaking the ionizable head group and the linker region in between. The work was grueling, and lipids that made great nanoparticles in a petri dish would often flop in animal studies. “You can have 50 different ionizable lipids that all deliver effectively to cells in culture, and 49 of them won’t work a damn in vivo,” recalls Thomas Madden, who worked at Inex and is now CEO of Acuitas Therapeutics.
LNPs take advantage of a natural process called receptor-mediated endocytosis to get into cells, Madden explains. Upon binding to a cell, the nanoparticle becomes encapsulated in an even bigger lipid bubble—an organelle called an endosome. The endosome’s acidic interior protonates the heads of the ionizable lipids, making them positively charged. That positive charge triggers a change in the shape of the nanoparticle, which scientists think helps it break free from the endosome and ultimately release its RNA cargo into the cell’s cytoplasm. Once released, the RNA is free to do its job.
The most effective nanoparticles were ones that the body mistook as low-density lipoprotein (LDL) cholesterol—commonly called bad cholesterol. Proteins that recognize LDL cholesterol in the blood bound to some of Alnylam’s nanoparticles and carried them to LDL receptors on liver cells, which then caused the cells to engulf the nanoparticles in an endosome. It was the kind of complex interplay that studies in a petri dish missed.

“A lot of work has gone into studying what happens inside a cell, but trying to understand the transport that occurs before these nanoparticles reach their cells is another question entirely,” says Kathryn Whitehead, a nanoparticle scientist at Carnegie Mellon University. As a consequence, “we don’t even screen in vitro anymore,” she says. “I find it more informative to test directly in an animal.”
Even some of the LNPs that worked well in animals proved too toxic for the repeated dosing required of many siRNA therapies. “The biggest issue was trying to find the right balance between systems that were effective but also safe and tolerable,” says Marian Gindy, executive director of pharmaceutical sciences at Merck & Co., who led the RNA formulation team from 2008 until Merck ended its siRNA programs in 2013. “And I would say that is still the biggest challenge in this area.”
Advertisement
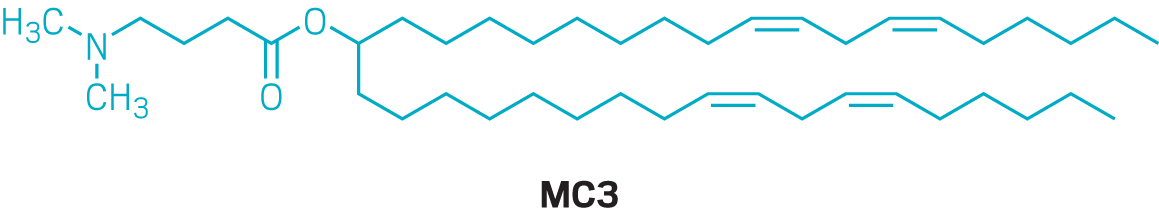
By 2010, Alnylam had landed on a winning ionizable lipid known as MC3. Nanoparticles based on MC3 required about one-thousandth the dose of LNPs made using older ionizable lipids. Alnylam used the new formulation in patisiran (Onpattro), its treatment for a rare disease called hereditary transthyretin-mediated amyloidosis. In 2018, patisiran became the first approved siRNA drug and the first approved therapy delivered via LNPs. But the drug requires an 80 min infusion every 3 weeks and pretreatment with multiple anti-inflammatory drugs to minimize reactions to the nanoparticle. By the time patisiran was showing promise in the clinic, Alnylam had set most of its LNP work aside in favor of a new chemical conjugation technology that it used to deliver its other siRNA therapies subcutaneously.
A launchpad for mRNA
For a brief time, new work on LNPs fell out of favor—that is, until new companies that were focused on mRNA brought fresh energy to the field. BioNTech, founded in 2008, and Moderna, founded in 2010, promised to be able to use mRNA to produce any protein in the body, as either a therapeutic or a vaccine. In the past decade, mRNA garnered billions of dollars of investment. Discovering how to deliver those mRNA strands into cells was a problem from day 1, but prior experience with siRNA provided a launching pad.
“Early on people recognized that the same lipids used for siRNA could also be useful for mRNA,” says Daniel Anderson, a nanomedicine and biomaterials scientist at MIT. His group began collaborating with the rare-disease company Shire Pharmaceuticals to encapsulate mRNA that encoded protein therapies to treat rare liver diseases.
The off-the-shelf LNP formulations designed for siRNA worked for mRNA occasionally but not very well, says Romesh Subramanian, who led a team at Alexion Pharmaceuticals that worked on mRNA therapies with Moderna from 2014 to 2017. siRNA molecules are like short rods, with two rows of about 20 nucleotides each, he explains. mRNA, in contrast, can easily span thousands of nucleotides, wind into complex shapes, and change the properties of the LNP in ways that are hard to predict.
After realizing that MC3 wouldn’t cut it for mRNA delivery, Moderna invested significant resources into building a better ionizable lipid. “There was a group of chemists put on this right away to build novel cationic lipids,” says Ciaramella, the former head of infectious diseases at Moderna. “It is kind of like a small-molecule drug discovery engine, but on steroids.” The team made about 100 ionizable lipids and introduced ester linkages into the carbon chains of the lipids to help make them more biodegradable, he recalls. Tweaking the ratio of the four lipids in the nanoparticles altered the LNPs’ distribution in the body. “The devil is absolutely in the details as far as LNPs are concerned,” Ciaramella says. “But once you optimize it for one organ, you can change out the mRNA with minimal optimization.”
That adaptability is key. For example, Moderna recently made an updated version of its COVID-19 vaccine for a new variant of the coronavirus first identified in South Africa. In that vaccine, which must still undergo clinical testing, the mRNA code is slightly changed to match the genetic code of the new strain of the virus, but the LNP formulation remains the same. Now that the company knows its nanoparticle works, it can use it over and over again for different vaccines.
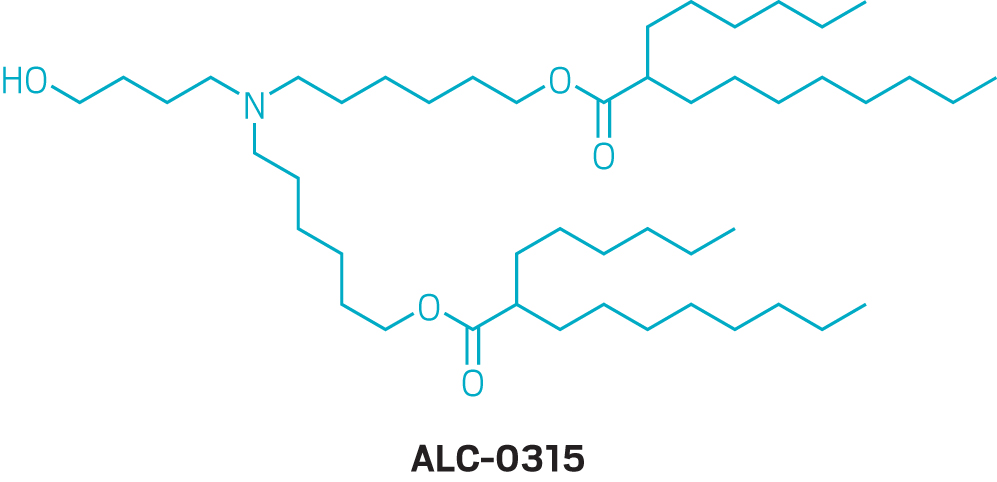
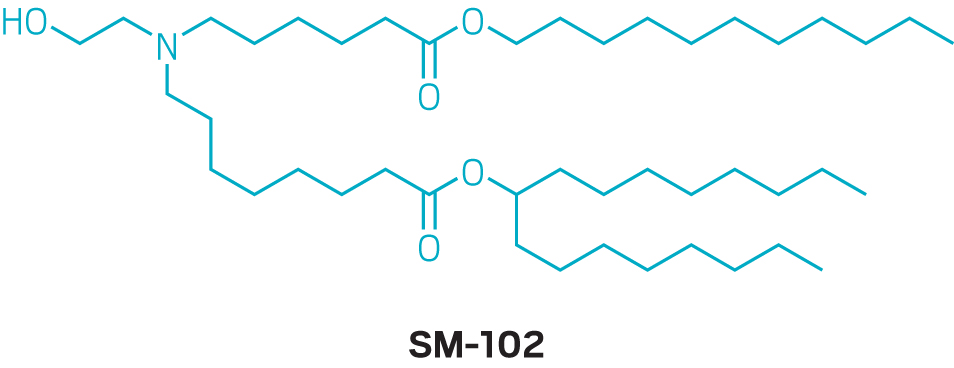
But details on how Moderna arrived at its optimal formulation in the first place are scant. The company did not grant an interview to talk about its nanoparticle development, and neither did Pfizer or BioNTech. For its COVID-19 vaccine, Moderna ultimately used an ionizable lipid that it calls SM-102, which it first described in a 2018 study on alternatives to MC3. Pfizer and BioNTech licensed an ionizable lipid called ALC-0315 from Acuitas.
Those ionizable lipids, which are remarkably similar in structure, were discovered while the firms were optimizing LNPs for systemic administration and delivery to the liver—not the intramuscular injection of a vaccine. Experts point out that optimizing the nanoparticles for vaccination could lead to shots that require lower doses, which could ease the manufacturing burden amid a pandemic. New lipids and nanoparticle formulations will likely take too long to develop to make a difference during this pandemic, but Moderna, BioNTech, and others are continuing to look for better ways to get mRNA into cells for a variety of applications.
An LNP resurgence
The pandemic has reinvigorated interest in continuing to refine LNPs. Small firms dedicated to the nanoparticles are getting more calls from larger drug companies that want to use their lipids. Effective LNPs could be crucial for new mRNA vaccines, mRNA therapies, DNA gene therapies, and even CRISPR gene-editing thrapies.
“Everyone is trying to figure out the next big ionizable lipid,” says Gaurav Sahay, a nanoparticle scientist at Oregon State University.. But he thinks that nanoparticle researchers should also start paying more attention to the other components of an LNP. Sahay says his lab found that using alternative versions of cholesterol molecules could dramatically improve delivery. And although both the Moderna and Pfizer-BioNTech vaccines use the same standard phospholipid, swapping out this ingredient for a different phospholipid could lead to nanoparticles that reach different cells in the body, Whitehead says.
“Delivery into specific cell and tissue populations is still a huge challenge for the field,” says Yizhou Dong, a nanoparticle researcher at the Ohio State University. Right now, intravenous injections of nanoparticles can easily reach the liver, and intramuscular injections for vaccines are taken up by immune cells. Some companies are working on experimental formulations for aerosolized delivery to the lungs, but the rest of the body remains out of reach, and the demand for targeted delivery is high. In late February, the CRISPR base-editing company Beam Therapeutics, where Ciaramella is president and chief scientific officer, paid $120 million to acquire the start-up Guide Therapeutics, which is focused on LNP discovery and has a system for finding particles that target specific cells of the body.
“There was this time when LNPs went through the dark ages,” says Thomas Barnes, CEO of the mRNA company Orna Therapeutics. “I think there is going to be a bit of a renaissance in these ionizable lipids and that the world is going to get excited about LNPs again.”
For now, the success of the COVID-19 vaccines is a sweet victory. “It is a little surreal, honestly,” Anderson says. “It is something that we went from just being excited about to something that my mom got in a shot in January.”


Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter