CHEMISTRY TAKES A
HOLIDAY
From space-age composites that form passenger jets to new, eco-friendly sunscreens, chemicals are upgrading vacation.
Illustration by Alexander Wells
Richardson's latest passion is river diving and underwater fossil hunting, and that's where her unusual packing list comes in. The screwdriver keeps her anchored to the riverbed, while the underwater light helps her zero in on interesting finds that she pops into her goody bag.
Vacations rejuvenate travelers, taking them away from the everyday world. Richardson finds that escape while immersed in blue-green waters inhabited by silvery salmon and trout that dart among reeds and algae. “It is usually silent—you just hear yourself breathe,” she explains.
For Richardson and many like her, vacations are about pursuing passions. For others, relaxation on a cruise is the name of the game. Many more enjoy exploring the great cities of the world. But no matter what kind of vacation travelers like, it's pleasure they're after, and they can thank chemistry for making it happen.
Composites take flight
Many vacations begin with an airplane flight. Chemistry is essential in lifting every jet off the ground. Most airplanes, though, are made of the same materials that were in vogue at the dawn of the jet age.
But novel new materials, such as lightweight polymer composites, will soon rule the sky. The Boeing 787 Dreamliner, for example, consists of nearly 50% composites by mass, which helps it reduce carbon dioxide emissions and save fuel.
Ultrastrong composites, like those in the Dreamliner, combine a polymer matrix derived from thermosetting resins and reinforcements made of materials such as carbon fibers, glass fibers, or aramid fibers (such as Kevlar).
Despite their advantages, composites are limited by operational temperatures, explains Boris Bulgakov of Lomonosov Moscow State University. “For most common materials in aerospace based on epoxy resins, operational temperatures cannot be higher than 120 °C; the usual range is −50 to 50 °C,“ he says. Some models of aircraft and parts, such as low-pressure compressor blades, can reach up to 350 °C.
Bulgakov and his team are developing alternative polymer matrices composed of bis-phthalonitriles, organic compounds with high aromatic content. Thermosetting plastics, like phthalonitrile resins, can be molded into various shapes then hardened with heat—but only once. Reheating the resins won't make them pliable again. That means phthalonitrile resins can be exposed to high operating temperatures without damage.
Although these thermosetting plastics have been around since the 1980s, they haven't taken off because the monomers' high melting points limited their use. Attempts to decrease the resins' melting points resulted in a decrease in thermoset properties, Bulgakov explains.
Now, his team has produced low-melting-point phthalonitrile resins with strong thermoset qualities. “Our resins can withstand temperatures up to 450 °C,” he says. “Also, the matrices possess the highest flame-retardant properties among the known plastics.”
Another perplexing issue for composites is bonding. Composites are built from multiple layers of material. And while they often have higher rigidity and greater strength, they are also subject to catastrophic failure.
A team from the Department of Aeronautics & Astronautics at Massachusetts Institute of Technology, working with Swedish aerospace company Saab, has devised a surprising solution: stitching composites with carbon nanotubes. It achieved this ultrastrong stitching by vertically aligning billions of carbon nanotubes per square centimeter of composite and bonding them with polymers.
The idea of boarding a plane that's “sewn together” may be unnerving for some travelers. But the team found that their carbon nanotube stitches were 30% stronger than traditional bonding methods. “Carbon nanotubes have an extremely high surface area—1,000 times more than carbon fiber,” explains team member Roberto Guzman, now a researcher at FIDAMC, the Foundation for the Research, Development & Application of Composite Materials in Madrid. “The greater the surface area, the higher the energy required to break the material.“
These new resins and bonding strategies may lead to composites that can be used in more and more extreme aerospace environments. That should lead to lighter-weight airplanes with lower emissions and fuel consumption. Travelers may not notice that from the (relative) comfort of seat 22C, but they may see it in lower ticket prices, while the more eco-minded passengers will also be cognizant of the smaller carbon footprint they're leaving.
Pooling our health
Richardson will never forget her first dive, off West Palm Beach, Fla. “It was incredible seeing the fish, the coral reefs, and all the color,” she says. “I instantly fell in love.”
Many divers take that first plunge on vacation. With 70% of Earth's surface covered in water, there is a smorgasbord of diving hot spots to pick from, ranging from the Amalfi Coast in Italy to the Great Blue Hole in Belize or Barracuda Point in Malaysia.
Despite that enviable experience, Richardson first earned her scuba diving stripes in the safety of a nearby swimming pool. For others, pools offer the opportunity to simply cool off, float down a resort's lazy river, or get soaked at a splash park. That means sharing the water with other vacationers. With all those people using them, how do public pools stay clean?
Initially, pools were cleaned by filtering out debris and regularly changing the water. That, of course, was hard, inefficient work. Brown University student John Wymond Miller Bunker swam to the rescue in 1911 in what is believed to be the first chemical attempt at cleaning a pool with chlorine. Inspired by city waterworks, where engineers were introducing chlorine and filtration systems to sterilize water, Bunker tried adding chlorine to a swimming pool. His results were sparkling and refreshing: Bacterial counts dropped from 700 per cubic centimeter to zero in just 15 minutes.
Chlorine-based cleaning chemicals like hypochlorous acid kill pathogens by breaking down their cell walls and destroying the proteins inside. It remains the most popular method of keeping pools sparkling clean.
There are now other options: Lovers of hot tubs can splash in some bromine, which works similar to chlorine but remains more stable in warm water. For a complete nonchemical solution, some pools are installed with high-intensity ultraviolet light filtration systems, which blast microorganisms with UV beams, destroying their DNA.
Tanks for the memories
Growing up in the heyday of Jacques Cousteau TV shows, Karl Shreeves became so captivated by the wonders of the underwater world that he became a certified diver by 13—the youngest age allowed at the time. “They say you'll never forget your first kiss,” says Shreeves. “But I found that while you do forget your first kiss, you won't forget the first time you breathe underwater.” Today, he dives all over the world, both on vacation and in his role as Education and Content Development Executive at the Professional Association of Diving Instructors.
For much of diving, divers rely on regular compressed air, a mixture of 21% oxygen and 79% nitrogen. But once a diver descends past a certain depth, that nitrogen can become toxic. Under greater pressure, the nitrogen dissolves into body tissues. “Nitrogen gets in your lungs and in your bloodstream,” Shreeves explains.
Once the pressure is removed by ascending, the absorbed nitrogen can form bubbles and cause decompression sickness, or “the bends.“ This is prevented by using dive computers that tell divers how long they can stay at various depths before stops are required to keep dissolved nitrogen within accepted limits while ascending.
An avid diver himself, Shreeves has some advice for those who want to dive at greater depths. To help divers stay longer, they can use enriched air mixtures (also called “nitrox”). “Enriched air commonly has 32% or 36% oxygen,” Shreeves explains. “We do this not for more oxygen, but less nitrogen.” This enables longer dive times without decompression stops.
Although it's not difficult to breathe nitrox and other gases used for much deeper diving, divers must understand the underlying chemistry. “Oxygen starts to become toxic under increasing pressure beyond certain depths,” says Shreeves. “So divers learn how oxygen-based limits work and why.“
Sunshine and a bright clear bay
The sun's UV rays beat down on land and scatter into water. Looking to shield themselves, vacationers lather on sunscreen. But in saving their skins, these travelers may be damaging marine environments.
The synthetic ingredients many sunscreens use to filter and absorb UV can pose a hazard to ocean wildlife. Several studies, in fact, found that these chemicals can contribute to bleaching and deformities in coral reefs. Hawaii passed a bill this year to ban sunscreens containing oxybenzone and octinoxate. Some manufacturers, meanwhile, now offer natural mineral alternatives that protect revelers and reefs.
Lifeguard Brian Guadagno founded the sunscreen line Raw Elements in his kitchen with the aim of producing eco-safe sunscreens. His mineral formulations use zinc oxide and protect the skin in a different way. Whereas chemical sunscreens, such as those using oxybenzone and octinoxate, are absorbed into the skin, mineral sunscreens physically block UV rays by sitting on the skin's surface, reflecting the rays.
Zinc oxide is a photocatalyst, and UV exposure can produce free radicals that may damage living cells. Therefore, Guadagno uses formulas which are rendered inert and are not photocatalysts. And, unlike nanoparticles, they cannot be ingested by coral or absorbed into the human bloodstream.
And critically, these more eco-conscious sunscreens will not leave beachgoers any more exposed, as mineral-based sunscreens can effectively protect skin from the whole UV spectrum.
Keeping it clean and green
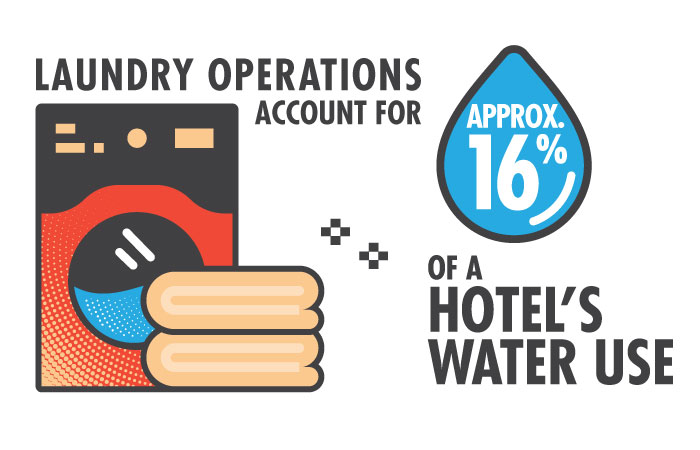
After relaxing in the sun, few things feel better than showering off the sand, drying with a fluffy towel, then falling asleep in crisp white sheets. Hotels offer such indulgences, but the resultant laundry operations use lots of resources. In fact, they account for approximately 16% of a given property's water use, according to the U.S. Environmental Protection Agency.
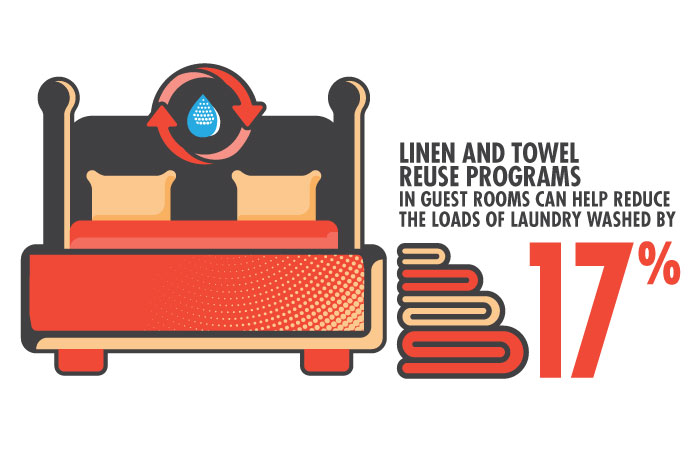
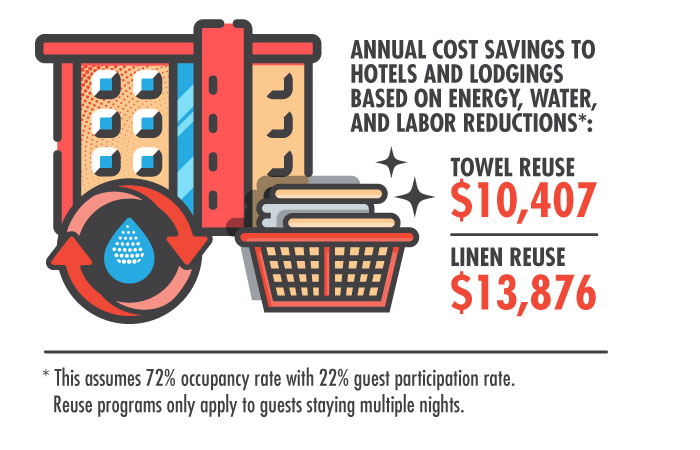
One of the simplest and most effective ways of reducing a hotel's energy, water, and detergent consumption is by persuading guests to reuse towels and sheets. To that end, the EPA launched the WaterSense H2Otel Challenge in 2014 to help hotels and guests conserve water. “According to the American Hotel & Lodging Association, a typical 300-room hotel can reduce its yearly water usage by 51,840 gallons and detergent usage by 346 gallons with a towel and linen reuse program,“ notes EPA spokesperson Enesta Jones.
Approximately 94% of hotels have implemented some form of reuse program, according to the American Hotel & Lodging Association. The Radisson Hotel Group (RHG) has even managed to link its reuse program to a bonus social benefit: access to safe drinking water. For every 500 towels reused, RHG makes a donation to the international water charity Just a Drop. Over 3 million towels have been reused to date.
Unpacking the chemistry of souvenirs
Sadly, all vacations must end. Before then, though—no matter how they spend their downtime—most vacationers will pick up souvenirs. In Richardson's case, her favorite souvenir was something she found on a riverbed: a nonfossilized horse jaw complete with three molars. “My curiosity got the best of me,” Richardson says. “I had to have it dated, since I thought it was from the Revolutionary War.” She sent it to the Woods Hole Oceanographic Institute, which confirmed her suspicions with mass spectrometry.
Richardson's unconventional souvenirs have also included “log dogs”—pieces of metal with a loop that 18th-century early settlers used to hook trees together and float them downriver. However, once exposed to air, these fragile artifacts start to disintegrate. Her diving buddy Jimmy, an accountant and self-taught electrochemist, stepped in to save the day. “He made his own electrochemistry rig to de-rust our log dogs and preserve them,” she says.
Chemistry has not only helped Richardson make vacation memories; the science has also enabled her to keep them for years to come.
Precipitating the conversation:
A Perspective from Randal Perry, Technical Fellow at The Chemours Company

You'll hear people complain that things aren't the way they used to be. When it comes to vacations, we can all be grateful for that. Road trips, such as the ones I took as a kid, were often marred by breakdowns, but now chemistry has brought us new materials that make cars—and other forms of transportation—much more reliable. Yesterday's cumbersome clothing and gear are now lighter, more capable, and more comfortable. Just about every part of vacationing is better now—except for airplane legroom—and it's all because of chemistry.
Chemistry has even made a difference when it comes to poolside fun. Splashing around in the water might seem like awfully low-tech recreation. But were it not for the materials revolution, you'd still be hiking up your wool swimsuit when you got out of the water.
Chlorine, meanwhile, prevents pool water from breeding disease. That's nothing new—the chemical's been used this way for over a hundred years. There have been some big changes, though, in how we produce it. Chlorine is isolated from brine via the chlor-alkali process, which also produces sodium hydroxide, or caustic lye. This process used to eat up lots of energy and involved some dangerous materials, like asbestos and mercury. These days, Nafion™ ion-selective membranes make the whole procedure significantly safer and more energy efficient.
That same chlorine that keeps us healthy in the pool also helps safeguard our drinking water. Well-chlorinated water can mean the difference between a great vacation and one spent at pit stops battling stomach cramps. Caustic lye—the other product of the chlor-alkali process—also makes our vacations better. It plays a large role in food preparation, including giving pretzels their distinctive taste, improving the texture of ice cream, and making chocolate so delicious.
And when it comes to getting you to your destination, Nafion™ will soon play a greater role there too. While mainstream fuel-cell vehicles are still some years off, their power packs will depend on Nafion™ to turn hydrogen into the electricity that powers the wheels. If you currently drive an electric vehicle, know that you may soon be charging it using large fluid batteries with Nafion™ membranes.
But don't think about any of this on vacation. I certainly don't. However, I do give Nafion™ a quiet thank-you whenever I find myself poolside at a resort, indulging in some chocolate or ice cream.
READ ABOUT HOW CHEMOURS HELPS MAKE AIR TRAVEL SAFER AND MORE FUN:
October 1: The Chemistry of Sports
November 26: The Chemistry of Travel
December 17: The Chemistry of Dining
HOW CHEMISTRY ROCKS MUSIC FESTIVALS
PACKING THE RIGHT MOLECULES FOR THE GREAT OUTDOORS
HOW CHEMISTRY THRILLS IN AMUSEMENT PARKS
CHEMISTRY ON ICE
ABOUT SPONSORED CONTENT
Sponsored content is not written by and does not necessarily reflect the views of C&EN's editorial staff. It is authored by writers approved by the C&EN BrandLab and held to C&EN's editorial standards, with the intent of providing valuable information to C&EN readers. This sponsored content feature has been produced with funding support from The Chemours Company.