Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Nanomaterials
Emilie Ringe
Plasmonics pioneer is harnessing the potential of magnesium nanoparticles
by Mark Peplow, special to C&EN
August 20, 2021
| A version of this story appeared in
Volume 99, Issue 30

Credit: Tommy Lavergne (Ringe); J. Phys. Chem. C. (nanoparticle shapes and plasmon maps); Shutterstock (magnesium tile)
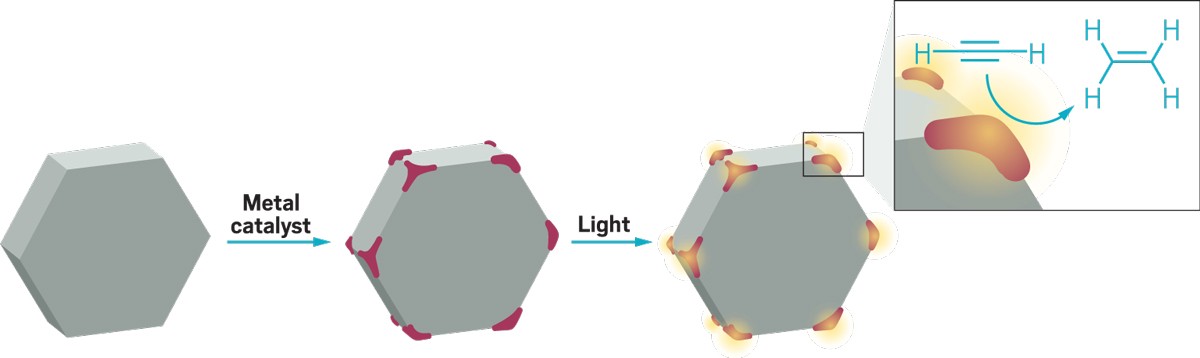
In 2018, Emilie Ringe had a pivotal year in her work with plasmonic nanoparticles. These tiny specks of matter act like antennas for light, gathering and concentrating energy that can be used to trigger chemical reactions or kill cancerous cells. The nanoparticles are often made from expensive metals like gold and silver, but Ringe discovered a way to use cheap and abundant magnesium in their place. “It was the start of most of the things we do now,” says Ringe, a lecturer at the University of Cambridge.
A plasmonic nanoparticle is a stiff lattice of positive ions in a sea of electrons, she explains. When light of just the right frequency hits the particle, it sets the electrons in resonant motion—like a parent pushing a child higher and higher on a playground swing. “These electrons sloshing backwards and forwards are superefficient at capturing light,” Ringe says. The particles focus energy so they can drive chemical reactions and other processes at their surface. “You can boil water with these particles just by shining light on them,” she says.
Advertisement
Very few elements in the periodic table possess this plasmonic prowess, and the usual suspects have some disadvantages. For many industrial applications, gold nanoparticles are still considered far too pricey, silver degrades too quickly, and aluminum resonates well only in response to ultraviolet light.
Ringe showed that magnesium could form effective plasmonic nanoparticles. Different sizes and shapes of magnesium particles absorb light from different parts of the spectrum from UV to visible and infrared, making them versatile as well as cheap.
Other researchers have taken some convincing that nanoparticles of a reactive element are stable. “Everyone always tells us, ‘It’s going to blow up in your face,’ ” Ringe says. Fortunately, the particles form a thin oxide layer that makes them stable in air for weeks. Her team is already decorating the plasmonic magnesium nanoparticles with other metals to test their photocatalytic abilities. Since the energy harvested by the nanoparticles eventually turns into heat, the particles could also be used to kill tumor cells in a strategy known as photothermal therapy.
Ringe grew up in Quebec and first worked on plasmonic nanoparticles during her PhD at Northwestern University, where she combined electron microscopy and spectroscopy to understand how the particles’ shapes affected their plasmonic properties. “She had an amazing number of publications, and awards all over the place,” says Laurence Marks of Northwestern, one of her PhD supervisors. He adds that her work already stands out in the field of nanoplasmonics. “She’s certainly one of the young stars,” he says. After a postdoc at Cambridge, Ringe moved to an assistant professorship at Rice University, where she made her magnesium discovery.
Back at Cambridge since 2018, Ringe has a dual appointment in materials science and earth sciences and is involved in microscopy and spectroscopy at every scale from millimeters to nanometers, studying natural and synthetic materials. While she hopes to forge links with industry to develop applications for her magnesium nanoparticles in the coming years, she is still prospecting for other elements that could expand the nanoplasmonic toolbox. “The periodic table is not quite done yet,” she says.
Vitals
Current affiliation: University of Cambridge
Age: 37
PhD alma mater: Northwestern University
Hometown: Montreal
If I were an element, I’d be: Magnesium. "I strive to be versatile and useful in new ways to many people and fields, just like magnesium plays key roles across biology, physics, and engineering.”
Role model: “One of my uncles. He runs a farm where I spent several weeks every summer. His consistent hard work, planning, and understanding of the natural world around him inspire me to keep going and make the world a better place.”
In 2018, Emilie Ringe had a pivotal year in her work with plasmonic nanoparticles. These tiny specks of matter act like antennas for light, gathering and concentrating energy that can be used to trigger chemical reactions or kill cancerous cells. The nanoparticles are often made from expensive metals like gold and silver, but Ringe discovered a way to use cheap and abundant magnesium in their place. “It was the start of most of the things we do now,” says Ringe, a lecturer at the University of Cambridge.
A plasmonic nanoparticle is a stiff lattice of positive ions in a sea of electrons, she explains. When light of just the right frequency hits the particle, it sets the electrons in resonant motion—like a parent pushing a child higher and higher on a playground swing. “These electrons sloshing backwards and forwards are superefficient at capturing light,” Ringe says. The particles focus energy so they can drive chemical reactions and other processes at their surface. “You can boil water with these particles just by shining light on them,” she says.
Very few elements in the periodic table possess this plasmonic prowess, and the usual suspects have some disadvantages. For many industrial applications, gold nanoparticles are still considered far too pricey, silver degrades too quickly, and aluminum resonates well only in response to ultraviolet light.
Vitals
▸ Current affiliation: University of Cambridge
▸ Age: 37
▸ PhD alma mater: Northwestern University
▸ Hometown: Montreal
▸ If I were an element, I’d be: Magnesium. "I strive to be versatile and useful in new ways to many people and fields, just like magnesium plays key roles across biology, physics, and engineering.”
▸ Role model: “One of my uncles. He runs a farm where I spent several weeks every summer. His consistent hard work, planning, and understanding of the natural world around him inspire me to keep going and make the world a better place.”
Ringe showed that magnesium could form effective plasmonic nanoparticles. Different sizes and shapes of magnesium particles absorb light from different parts of the spectrum from UV to visible and infrared, making them versatile as well as cheap.
Other researchers have taken some convincing that nanoparticles of a reactive element are stable. “Everyone always tells us, ‘It’s going to blow up in your face,’ ” Ringe says. Fortunately, the particles form a thin oxide layer that makes them stable in air for weeks. Her team is already decorating the plasmonic magnesium nanoparticles with other metals to test their photocatalytic abilities. Since the energy harvested by the nanoparticles eventually turns into heat, the particles could also be used to kill tumor cells in a strategy known as photothermal therapy.

Ringe grew up in Quebec and first worked on plasmonic nanoparticles during her PhD at Northwestern University, where she combined electron microscopy and spectroscopy to understand how the particles’ shapes affected their plasmonic properties. “She had an amazing number of publications, and awards all over the place,” says Laurence Marks of Northwestern, one of her PhD supervisors. He adds that her work already stands out in the field of nanoplasmonics. “She’s certainly one of the young stars,” he says. After a postdoc at Cambridge, Ringe moved to an assistant professorship at Rice University, where she made her magnesium discovery.
Back at Cambridge since 2018, Ringe has a dual appointment in materials science and earth sciences and is involved in microscopy and spectroscopy at every scale from millimeters to nanometers, studying natural and synthetic materials. While she hopes to forge links with industry to develop applications for her magnesium nanoparticles in the coming years, she is still prospecting for other elements that could expand the nanoplasmonic toolbox. “The periodic table is not quite done yet,” she says.

















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter