Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Pharmaceuticals
Shanique Borteley Alabi
Molecular matchmaker is designing drugs that break down badly behaving proteins
by Laura Howes
August 20, 2021
| A version of this story appeared in
Volume 99, Issue 30

Credit: Courtesy of Shanique Borteley Alabi (Alabi); Juan Gaertner/Science Source (3D structure); Science Picture Co/Science Source (cancer cell)
Taking out the trash isn’t everyone’s favorite chore, but for Shanique Borteley Alabi, our cellular garbage disposal could be the key to fighting disease. At Monte Rosa Therapeutics in Boston, she’s developing molecules to send cancer-causing proteins to the scrapyard.
Ask Alabi what piqued her interest in this chemical biology trickery, and she’ll tell you that she can’t remember a time when she wasn’t interested in drug discovery and how small molecules interact with the body. Alabi spent her childhood between the US and Ghana, and she says she found scientific inspiration in both places: for example, when helping out in the family pharmacy in Ghana or being inspired by “phenomenal” teachers in the US, like her high school science teacher Miss Gill.
Advertisement
When she started her PhD at Yale University, Alabi was drawn to projects at the interface between chemistry and biology, especially those using small molecules to get different proteins in the body to interact. She joined chemical biologist Craig Crews’s lab, where she helped design a compound that targets mutant forms of BRAF, a protein implicated in many cancers.
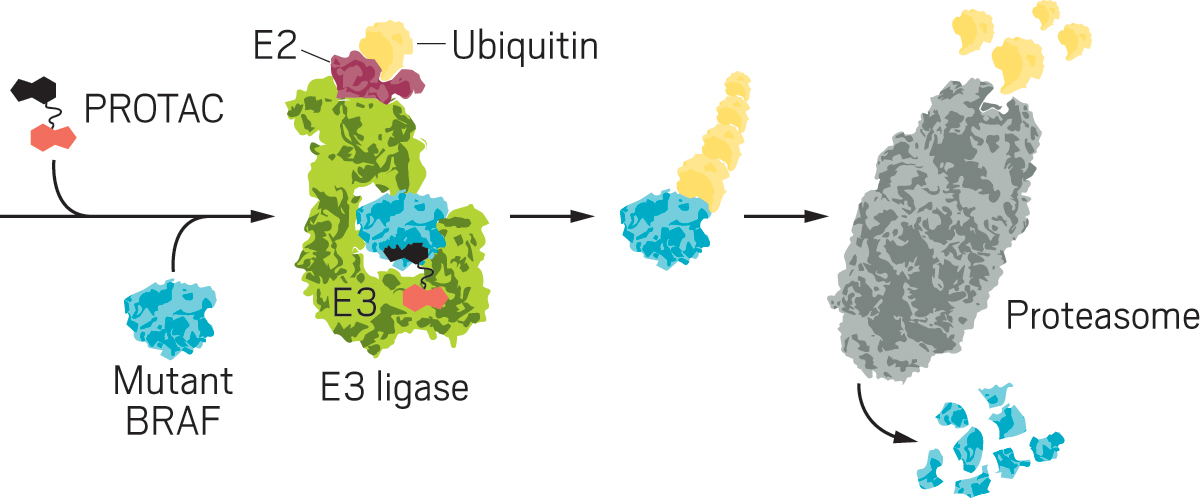
BRAF is part of a key signaling pathway that controls how cells grow and divide. When it stops working properly, it can lead to cancer, especially melanomas and colon cancer. Three BRAF-targeting drugs are on the market in the US, but they treat only cancers driven by one specific mutation in the protein. Meanwhile, hundreds of other mutations exist with no approved targeted therapies. Alabi and her colleagues decided to try a more general approach: disposing of the mutated proteins altogether. They developed small molecules called PROTACs (proteolysis-targeting chimeras), bifunctional molecules that bind at one end to a protein of interest and at the other end to an E3 ligase, an enzyme that causes a ubiquitin to be tacked on to the target protein. The ubiquitin labels the protein for disposal in the proteasome, a big, trash-can-shaped enzyme that chomps up proteins.
Alabi devised a breakthrough idea: use vemurafenib, one of the drugs that targets BRAF, as the basis of a PROTAC. Vemurafenib is known to bind to many BRAF mutants but not inhibit them. In contrast, the vemurafenib-based protein degrader, SJF-0628, induces degradation of all classes of BRAF mutants.
In addition to offering a potential new therapeutic for BRAF, the work shows that you can selectively target all cancer-causing mutants without affecting the nonmutant form. The prospect of broad-acting, safe degraders has people excited, Crews says. He says it was Alabi’s grit and determination leading the project that singled her out as a scientist going places. “Shanique is not only an excellent researcher, but she also thinks strategically,” Crews says. “I’m confident that she can be very successful.”
Leaders at Monte Rosa obviously think so too. She started with the biotech in January 2021—the month after defending her PhD. While Alabi worked purely on PROTAC development during her PhD, her focus has now switched to the related, but not identical, field of molecular glues. These molecules induce protein degradation through a different mechanism. And that means the chemistry is also different.
To be targeted by a PROTAC, a protein needs to have a pocket or handle for a small molecule—a feature many disease-causing proteins lack. In contrast, Alabi explains, molecular glues, just like regular glues, sit between surfaces to stick them together. The molecule first slides onto part of the E3 ligase, smoothing itself along the surface. The molecular glue causes the shape and chemistry of the ligase to change, which draws in the target protein. Researchers hope the approach will allow them to access a wider range of difficult-to-drug proteins.
Alabi says she has already learned a lot about the collaborative style of working in a biotech, and in a few short months in industry, she has defined her career goal: to eventually lead drug discovery at a company.
Vitals
Current affiliation: Monte Rosa Therapeutics
Age: 29
PhD alma mater: Yale University
Hometown: Bristow, Virginia
If I were an element, I’d be: Gold. “It best symbolizes my heritage from Ghana, the former Gold Coast, and I love gold jewelry!”
Role model: “I greatly admire Kizzmekia Corbett, whose research on the spike protein of coronaviruses was vital to the development of the COVID-19 vaccine.”
Taking out the trash isn’t everyone’s favorite chore, but for Shanique Borteley Alabi, our cellular garbage disposal could be the key to fighting disease. At Monte Rosa Therapeutics in Boston, she’s developing molecules to send cancer-causing proteins to the scrapyard.
Ask Alabi what piqued her interest in this chemical biology trickery, and she’ll tell you that she can’t remember a time when she wasn’t interested in drug discovery and how small molecules interact with the body. Alabi spent her childhood between the US and Ghana, and she says she found scientific inspiration in both places: for example, when helping out in the family pharmacy in Ghana or being inspired by “phenomenal” teachers in the US, like her high school science teacher Miss Gill.
When she started her PhD at Yale University, Alabi was drawn to projects at the interface between chemistry and biology, especially those using small molecules to get different proteins in the body to interact. She joined chemical biologist Craig Crews’s lab, where she helped design a compound that targets mutant forms of BRAF, a protein implicated in many cancers.
Vitals
▸ Current affiliation: Monte Rosa Therapeutics
▸ Age: 29
▸ PhD alma mater: Yale University
▸ Hometown: Bristow, Virginia
▸ If I were an element, I’d be: Gold. “It best symbolizes my heritage from Ghana, the former Gold Coast, and I love gold jewelry!”
▸ Role model: “I greatly admire Kizzmekia Corbett, whose research on the spike protein of coronaviruses was vital to the development of the COVID-19 vaccine.”
BRAF is part of a key signaling pathway that controls how cells grow and divide. When it stops working properly, it can lead to cancer, especially melanomas and colon cancer. Three BRAF-targeting drugs are on the market in the US, but they treat only cancers driven by one specific mutation in the protein. Meanwhile, hundreds of other mutations exist with no approved targeted therapies. Alabi and her colleagues decided to try a more general approach: disposing of the mutated proteins altogether. They developed small molecules called PROTACs (proteolysis-targeting chimeras), bifunctional molecules that bind at one end to a protein of interest and at the other end to an E3 ligase, an enzyme that causes a ubiquitin to be tacked on to the target protein. The ubiquitin labels the protein for disposal in the proteasome, a big, trash-can-shaped enzyme that chomps up proteins.
Alabi devised a breakthrough idea: use vemurafenib, one of the drugs that targets BRAF, as the basis of a PROTAC. Vemurafenib is known to bind to many BRAF mutants but not inhibit them. In contrast, the vemurafenib-based protein degrader, SJF-0628, induces degradation of all classes of BRAF mutants.

In addition to offering a potential new therapeutic for BRAF, the work shows that you can selectively target all cancer-causing mutants without affecting the nonmutant form. The prospect of broad-acting, safe degraders has people excited, Crews says. He says it was Alabi’s grit and determination leading the project that singled her out as a scientist going places. “Shanique is not only an excellent researcher, but she also thinks strategically,” Crews says. “I’m confident that she can be very successful.”
Leaders at Monte Rosa obviously think so too. She started with the biotech in January 2021—the month after defending her PhD. While Alabi worked purely on PROTAC development during her PhD, her focus has now switched to the related, but not identical, field of molecular glues. These molecules induce protein degradation through a different mechanism. And that means the chemistry is also different.
To be targeted by a PROTAC, a protein needs to have a pocket or handle for a small molecule—a feature many disease-causing proteins lack. In contrast, Alabi explains, molecular glues, just like regular glues, sit between surfaces to stick them together. The molecule first slides onto part of the E3 ligase, smoothing itself along the surface. The molecular glue causes the shape and chemistry of the ligase to change, which draws in the target protein. Researchers hope the approach will allow them to access a wider range of difficult-to-drug proteins.
Alabi says she has already learned a lot about the collaborative style of working in a biotech, and in a few short months in industry, she has defined her career goal: to eventually lead drug discovery at a company.

















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter