Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Energy Storage
Diego Solis-Ibarra
Perovskite powerhouse is perfecting this family of sunlight-absorbing semiconductors
by Mitch Jacoby
August 20, 2021
| A version of this story appeared in
Volume 99, Issue 30

Credit: Courtesy of Diego Solis-Ibarra (Solis-Ibarra); Angew. Chem., Int. Ed. (structure, perovskites)
Diego Solis-Ibarra can think pretty quickly on his feet. He can run darn fast on them too. So fast, in fact, that this materials chemist at the National Autonomous University of Mexico (UNAM) aspired to be an Olympic triathlete. “That was my dream when I was young,” he says. It was more than a flight of fancy. Solis-Ibarra participated in Pan-American triathlons and placed near the top of his age bracket in Mexico for a few years.
“I’m competitive by nature,” he says. He’s also pragmatic. “I was decent at the sport, but I knew I wasn’t good enough to make it to the Olympics.” So he focused instead on another one of his big interests—chemistry.
Advertisement
Early in his undergraduate days at the university where he is now a professor, Solis-Ibarra began learning about the world’s dependence on petroleum, global warming, and other environmental consequences of burning fossil fuels. He began studying about alternative forms of energy and was inspired by the potential benefits they offered.
“It really opened my eyes,” he says. “It made me wonder how I could use chemistry to address today’s pressing energy and environmental issues.”
As a patriotic citizen of sunny Mexico, he was especially taken with the promise of solar energy, a green way to capitalize on a resource abundant in his country. He recalls chatting with college friends about the possibility of making cheap photovoltaic solar panels that could generate electricity anywhere. “The idea seemed amazing to me, and it stuck in my mind.”
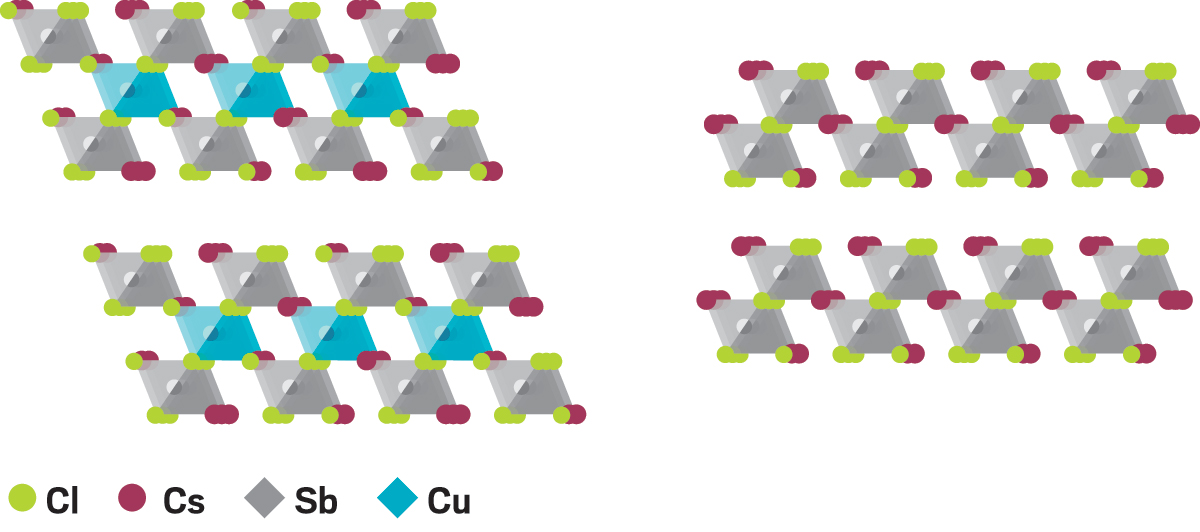
Years later, it’s still on his mind. These days, Solis-Ibarra runs a research group that focuses on solar cells containing perovskites, a family of sunlight-absorbing semiconductors. The popularity of these devices skyrocketed several years ago because their power conversion efficiency—a measure of sunlight in and electricity out—quickly reached that of crystalline silicon, the standard photovoltaic material, yet perovskites are much less expensive to make and process.
But several shortcomings have hindered manufacturing and commercialization of perovskite cells. For example, perovskites with the best power conversion efficiency values tend to decompose on exposure to moisture, heat, and intense light. They also contain lead, which is toxic.
Solis-Ibarra’s group is helping overcome those hurdles. In one study, his team came up with a way to replace lead with a combination of copper and antimony, both of which are nontoxic and abundant. Unlike single-metal substitutes, which yield poor photovoltaic performance, the mixed-metal compound stands up to heat, light, and humidity, and it conducts charge better than common perovskites.
In another study, the UNAM group gave 2D perovskites a much-needed shot in the arm. Compared with 3D perovskites, which are the common form, 2D analogs exhibit better stability when exposed to water and light, but they tend to be weak conductors. To boost conductivity, Solis-Ibarra and coworkers worked out a method for doping 2D perovskites with conjugated diynes and converting those groups to polydiacetylenes, which remain embedded in the perovskite structure. The modification made the 2D materials better light absorbers and jacked up the conductivity by a factor of 1,000.
Hemamala Karunadasa, Solis-Ibarra’s postdoc adviser at Stanford University, describes Solis-Ibarra as “a wonderful coworker and natural teacher.” She says his work on mixed-metal compounds has motivated groups around the world to expand into that line of research, adding, “Diego is a clever scientist who has come up with some very innovative chemistry.”
Vitals
Current affiliation: National Autonomous University of Mexico
Age: 36
PhD alma mater: National Autonomous University of Mexico
Hometown: Mexico City
If I were an element, I’d be: Vanadium. “First seen in Mexico, colorful, and loves multiple oxidation states.”
Role Model: “My dad. He taught me that everything has a solution if you are creative enough and are willing to put in the work to solve it.”
Diego Solis-Ibarra can think pretty quickly on his feet. He can run darn fast on them too. So fast, in fact, that this materials chemist at the National Autonomous University of Mexico (UNAM) aspired to be an Olympic triathlete. “That was my dream when I was young,” he says. It was more than a flight of fancy. Solis-Ibarra participated in Pan-American triathlons and placed near the top of his age bracket in Mexico for a few years.
“I’m competitive by nature,” he says. He’s also pragmatic. “I was decent at the sport, but I knew I wasn’t good enough to make it to the Olympics.” So he focused instead on another one of his big interests—chemistry.
Early in his undergraduate days at the university where he is now a professor, Solis-Ibarra began learning about the world’s dependence on petroleum, global warming, and other environmental consequences of burning fossil fuels. He began studying about alternative forms of energy and was inspired by the potential benefits they offered.
Vitals
▸ Current affiliation: National Autonomous University of Mexico
▸ Age: 36
▸ PhD alma mater: National Autonomous University of Mexico
▸ Hometown: Mexico City
▸ If I were an element, I’d be: Vanadium. “First seen in Mexico, colorful, and loves multiple oxidation states.”
▸ Role Model: “My dad. He taught me that everything has a solution if you are creative enough and are willing to put in the work to solve it.”
“It really opened my eyes,” he says. “It made me wonder how I could use chemistry to address today’s pressing energy and environmental issues.”
As a patriotic citizen of sunny Mexico, he was especially taken with the promise of solar energy, a green way to capitalize on a resource abundant in his country. He recalls chatting with college friends about the possibility of making cheap photovoltaic solar panels that could generate electricity anywhere. “The idea seemed amazing to me, and it stuck in my mind.”
Years later, it’s still on his mind. These days, Solis-Ibarra runs a research group that focuses on solar cells containing perovskites, a family of sunlight-absorbing semiconductors. The popularity of these devices skyrocketed several years ago because their power conversion efficiency—a measure of sunlight in and electricity out—quickly reached that of crystalline silicon, the standard photovoltaic material, yet perovskites are much less expensive to make and process.
But several shortcomings have hindered manufacturing and commercialization of perovskite cells. For example, perovskites with the best power conversion efficiency values tend to decompose on exposure to moisture, heat, and intense light. They also contain lead, which is toxic.

Solis-Ibarra’s group is helping overcome those hurdles. In one study, his team came up with a way to replace lead with a combination of copper and antimony, both of which are nontoxic and abundant. Unlike single-metal substitutes, which yield poor photovoltaic performance, the mixed-metal compound stands up to heat, light, and humidity, and it conducts charge better than common perovskites.
In another study, the UNAM group gave 2D perovskites a much-needed shot in the arm. Compared with 3D perovskites, which are the common form, 2D analogs exhibit better stability when exposed to water and light, but they tend to be weak conductors. To boost conductivity, Solis-Ibarra and coworkers worked out a method for doping 2D perovskites with conjugated diynes and converting those groups to polydiacetylenes, which remain embedded in the perovskite structure. The modification made the 2D materials better light absorbers and jacked up the conductivity by a factor of 1,000.
Hemamala Karunadasa, Solis-Ibarra’s postdoc adviser at Stanford University, describes Solis-Ibarra as “a wonderful coworker and natural teacher.” She says his work on mixed-metal compounds has motivated groups around the world to expand into that line of research, adding, “Diego is a clever scientist who has come up with some very innovative chemistry.”



















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter