Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Inorganic Chemistry
Robert J. Gilliard, Jr.
Main-group marvel is solving big problems with cheaper starting materials
by Leigh Krietsch Boerner
August 14, 2020
| A version of this story appeared in
Volume 98, Issue 31

Credit: Tom Cogill (Gilliard); Courtesy of Robert J. Gilliard, Jr. (pellets, crystals)
As a main-group chemist, Robert J. Gilliard, Jr. has made a career out of appreciating the underappreciated. He is devoted to exploring the chemistry that the underestimated middle children of the periodic table—elements like beryllium, bismuth, and germanium—can do.
“Historically, people haven’t thought that main-group elements could have similar properties to transition metals,” he says. Transition-metal compounds have been the go-to for catalysts and materials for years, but chemists have realized that main-group elements can sometimes substitute in for them. Now main-group elements are doing chemistry once thought impossible, Gilliard says.
Advertisement
One advantage of main-group elements is that they’re abundant and therefore inexpensive. Gilliard wields compounds made from certain s- and p-block elements to develop cheaper and more efficient energy conversion and storage technology, and to discover new chemistry.
Main-group chemistry is “so diverse in terms of the projects and problems that you’re able to tackle,” Gilliard says. “It has a huge impact in a lot of other areas of science.”
Gilliard’s own work jostles the impossible in a myriad of ways, such as using main-group alternatives for traditional transition-metal catalysts, which tend to be pricey and toxic. Recently, Gilliard and coworkers synthesized, isolated, and crystallized the first known stable beryllium radical cation, previously only seen on the surface of the sun and in the gas phase on Earth. Because the rare compound carries a low +1 oxidation state, it potentially could catalyze oxidative addition and reductive elimination, a job normally left to transition metals.
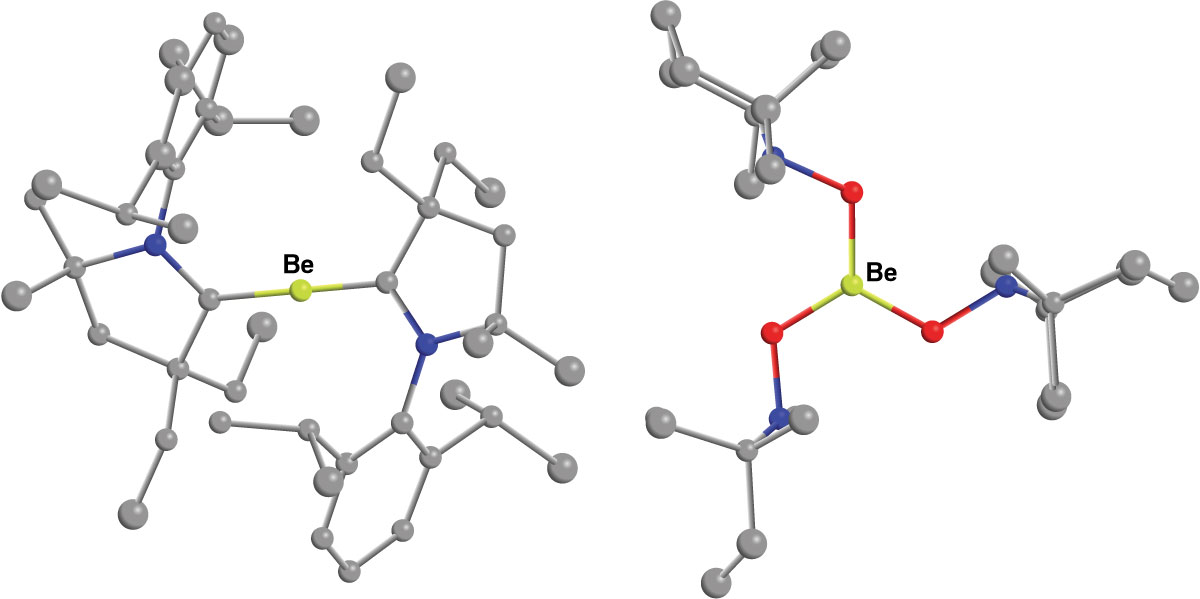
Being able to pin down and stabilize tricky-to-make compounds is a theme that runs through Gilliard’s research. His group’s projects swing across a wide range, including using carbenes and sodium or potassium to reduce CO2, tuning the optical properties of heterocycles with group 13 and 14 elements for energy-efficient light-based technologies, building magnesium-based reversible hydrogen storage materials, and using low-oxidation-state compounds to discover new redox properties. Much of this chemistry involves species that are hard to wrangle, such as radicals and hydrides.
“It’s very difficult chemistry, and he’s doing things that you’d expect it would take several years to build up the ability to do,” says Rhett Smith, an organic chemist at Clemson University and Gilliard’s undergraduate research adviser. Gilliard is 3 years into his initial appointment at the University of Virginia and has already published 16 papers in high-impact journals.
“Gilliard is a gifted synthetic chemist and an excellent mentor,” says John Protasiewicz, an organometallic chemist at Case Western Reserve University and one of Gilliard’s postdoctoral advisers. “He is going to become one of the big names in main-group chemistry.”
Gilliard points to his undergraduate research experience as critical to his career. Now the director of chemistry undergraduate research at UVA, 5 of the 14 members of his lab group are undergraduates. Being a mentor is one reason he wanted an academic job, he says. “I wanted to have an impact on others, and help others reach their goals.”
The other reason is the sheer joy of doing your own research, he says. “I like being able to dream and imagine things, particularly molecules and reactions, and then be able to do them as I see fit.”
Vitals
Current affiliation: University of Virginia
Age: 33
PhD alma mater: University of Georgia
Hometown: Hartsville, South Carolina
If I weren’t a chemist, I’d be: A lawyer. “As a chemist, educator, and researcher, I would like to think that I pursue truth, facts, and justice.”
If I were an element, I’d be: Bismuth. “It’s not the first metal you think about on the periodic table but it’s quite powerful and has unique properties compared to the other elements in group 15, including being relatively nontoxic.”
As a main-group chemist, Robert J. Gilliard, Jr. has made a career out of appreciating the underappreciated. He is devoted to exploring the chemistry that the underestimated middle children of the periodic table—elements like beryllium, bismuth, and germanium—can do.
Vitals
▸ Current affiliation: University of Virginia
▸ Age: 33
▸ PhD alma mater: University of Georgia
▸ Hometown: Hartsville, South Carolina
▸ If I weren’t a chemist, I’d be: A lawyer. “As a chemist, educator, and researcher, I would like to think that I pursue truth, facts, and justice.”
▸ If I were an element, I’d be: Bismuth. “It’s not the first metal you think about on the periodic table but it’s quite powerful and has unique properties compared to the other elements in group 15, including being relatively nontoxic.”
“Historically, people haven’t thought that main-group elements could have similar properties to transition metals,” he says. Transition-metal compounds have been the go-to for catalysts and materials for years, but chemists have realized that main-group elements can sometimes substitute in for them. Now main-group elements are doing chemistry once thought impossible, Gilliard says.
One advantage of main-group elements is that they’re abundant and therefore inexpensive. Gilliard wields compounds made from certain s- and p-block elements to develop cheaper and more efficient energy conversion and storage technology, and to discover new chemistry.
Main-group chemistry is “so diverse in terms of the projects and problems that you’re able to tackle,” Gilliard says. “It has a huge impact in a lot of other areas of science.”
Gilliard’s own work jostles the impossible in a myriad of ways, such as using main-group alternatives for traditional transition-metal catalysts, which tend to be pricey and toxic. Recently, Gilliard and coworkers synthesized, isolated, and crystallized the first known stable beryllium radical cation, previously only seen on the surface of the sun and in the gas phase on Earth. Because the rare compound carries a low +1 oxidation state, it potentially could catalyze oxidative addition and reductive elimination, a job normally left to transition metals.
Being able to pin down and stabilize tricky-to-make compounds is a theme that runs through Gilliard’s research. His group’s projects swing across a wide range, including using carbenes and sodium or potassium to reduce CO2, tuning the optical properties of heterocycles with group 13 and 14 elements for energy-efficient light-based technologies, building magnesium-based reversible hydrogen storage materials, and using low-oxidation-state compounds to discover new redox properties. Much of this chemistry involves species that are hard to wrangle, such as radicals and hydrides.
“It’s very difficult chemistry, and he’s doing things that you’d expect it would take several years to build up the ability to do,” says Rhett Smith, an organic chemist at Clemson University and Gilliard’s undergraduate research adviser. Gilliard is 3 years into his initial appointment at the University of Virginia and has already published 16 papers in high-impact journals.
“Gilliard is a gifted synthetic chemist and an excellent mentor,” says John Protasiewicz, an organometallic chemist at Case Western Reserve University and one of Gilliard’s postdoctoral advisers. “He is going to become one of the big names in main-group chemistry.”
Gilliard points to his undergraduate research experience as critical to his career. Now the director of chemistry undergraduate research at UVA, 5 of the 14 members of his lab group are undergraduates. Being a mentor is one reason he wanted an academic job, he says. “I wanted to have an impact on others, and help others reach their goals.”
The other reason is the sheer joy of doing your own research, he says. “I like being able to dream and imagine things, particularly molecules and reactions, and then be able to do them as I see fit.”


















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter