THE ELEMENTS OF
COOKING
Biryanis, barbecues, and kombucha—chemistry creates the sensory delights of global cuisine.
Illustration by Alexander Wells
Sharma, now 38, looks back fondly on those days. “I think it’s exciting to see something like that happen—to see something spontaneous and colorful change in front of you,” he says. “It almost feels magical.” The budding scientist pestered his parents until they caved in and bought him a chemistry kit.
Sharma’s playful explorations continued in his makeshift kitchen lab, where he blew up test tubes with exothermic reactions and discovered how displacement turned copper cookware blue. His childhood was immersed in an array of tastes and aromas. “There were Western influences as well as the more traditional Hindu influences,” he says.
Sharma went on to study medical biochemistry at college before moving to the U.S. and becoming a medical researcher in Washington, D.C. But his love affair with cooking endured.
Breathing academia day and night, Sharma found release writing a cooking blog, A Brown Table. It was a hit, reaching up to 20,000 readers a month. And when he and his husband later moved to San Francisco, the San Francisco Chronicle approached him to write three Sunday columns a month. Now a full-time chef and author, Sharma recently released his first cookbook, “Season: Big Flavors, Beautiful Food.”
Throughout his life, Sharma has been fascinated by the ways chemistry and cooking connect. “Even how recipes are laid out—they are like experiments,” he says. It is a perfect coupling that permeates kitchens around the world. The sensory delights of global cuisine, including Indian spices and probiotic Japanese brews, are the result of chemistry.
The joy of spices
For centuries, India’s cooks have blended spices like cardamom, cumin, anise, fenugreek, and coriander in creative ways. The result: a unique culinary tradition with multiple regional variations. “One of the things I found fascinating about spices was that, depending on the ratio of the mix, you can create a very different sensory effect,” Sharma explains.
Traveling around India, he saw how people experimented with garam masala, a standard Indian spice mix. “In the north, they cook for more aroma and use a higher ratio of cardamom,” he explains. “If you move south, they like it warmer, so they’ll add more cinnamon.”
Chemistry determines how these spices smell and taste, explains Krishnapura Srinivasan, a biochemist at the Central Food Technological Research Institute, in Mysore, India. “Specific spice-active compounds are responsible for imparting the typical flavor and aroma of individual spices.”
The capsaicin in chili pepper and piperine in black pepper produce the pungency, or spiciness, of these seasonings, while curcumin creates the distinct yellow color of turmeric. “The eugenol of cloves, the gingerol of ginger rhizomes, and the cuminaldehyde of cumin seeds are responsible for these spices’ characteristic aromas and flavors,” Srinivasan says.
Spiciness itself is not a taste. It’s a food characteristic in which chemicals, such as capsaicin, produce a neural signal, stimulating heat-sensitive neurons, known as nociceptors, in the mouth. These neurons transmit pain and heat sensations. The result: that familiar, intense, hot feeling.
The chemical constituents of spices can also produce pharmacological benefits, Srinivasan adds, and include antimicrobial, antioxidant, and anti-inflammatory properties. These healthy reactions are produced by the same chemicals that influence taste, such as eugenol, piperine, gingerol, and curcumin. “With the long history of the use of spices dating back to 5000 B.C., spices may be considered the first-ever functional food,” Srinivasan says, refering to edibles thought to have positive health effects beyond nutrition.
Bacterial brews
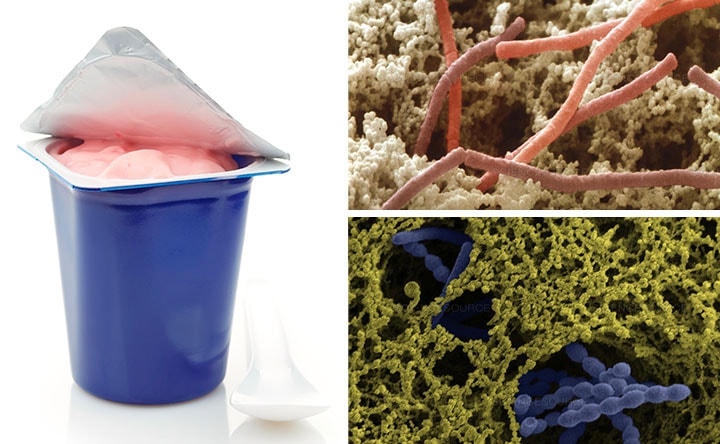
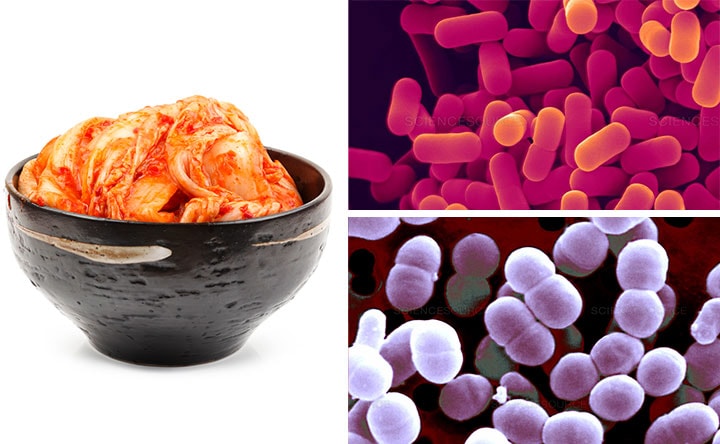
Like Indian culture, Japanese culture has long recognized a direct connection between food and well-being. Alongside classic dishes such as sushi and tempura, a Japanese tasting table features probiotic foods and drinks, such as miso and kombucha tea, which contain health-promoting microorganisms as a key ingredient.
Served as a somewhat sour, slightly effervescent brew, kombucha is based on a symbiotic culture of bacteria and yeast (SCOBY). Its production draws on ancient fermentation techniques that can be found throughout washoku, or traditional Japanese cuisine. Fermentation occurs when microorganisms anaerobically break down sugars or amino acids in substances; during the process, gases, acids, heat, and/or alcohol are produced. Soy sauce, for example, is blended from fermented soy beans, wheat, salt, and koji rice mold, whereas nattō comprises fermented soybeans and Bacillus subtilis bacteria.
Originally from China, kombucha has been consumed in Japan for thousands of years but is now popular with a global health-conscious public, who drinks it as a source of protein and antioxidants. As a probiotic, kombucha is also believed to improve a person’s gut microbiota by boosting the number of beneficial microorganisms in the mix.
In the 21st century, it is the chemistry of probiotics that excites many Japanese researchers. Food science in Japan is in a period of transition as it moves toward a greater focus on the molecular mechanisms that underpin these so-called functional foods, says Ikuo Kimura, a biology associate professor at Tokyo University of Agriculture & Technology.
“During food ingestion, small molecules produced through digestion and metabolism not only serve as energy sources but also signal transduction molecules to exert physiological functions via the nutrient-sensing receptors,” Kimura explains.
As researchers increase their understanding of these receptors, he believes we'll see more fermentation methods targeting food function at a molecular level and a growth in the organic synthesis and chemical modification of functional foods. Sipping on a mug of piping-hot bacteria has never seemed so appealing.
Sizzling up a maillard reaction
While Japanese and Indian cuisines are packed with distinctive ingredients that make them instantly recognizable, certain traits run through all cooking traditions, such as the use of flour, water, or rice. Likewise, we find commonality in how we pair foods.
When Sharma moved from India to the U.S., one of the first things he noticed was a shared love of combining meat with potatoes. “If you look at chicken biryani, a very traditional Muslim dish served in India, you see it is often created with fried potatoes on top and then garnished with onion,” he says. “You come to the West and you have mashed potatoes that are often served with a pork chop or steak. There are certain elements people repeat all over the globe, but they’re done in their own way,” he says.
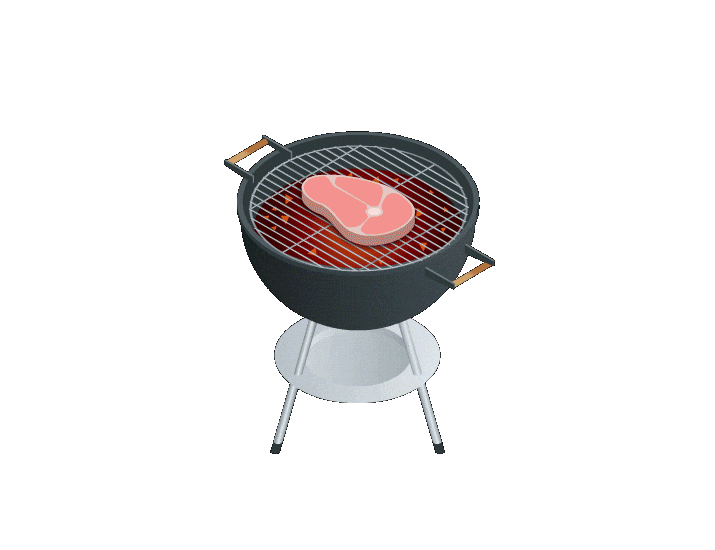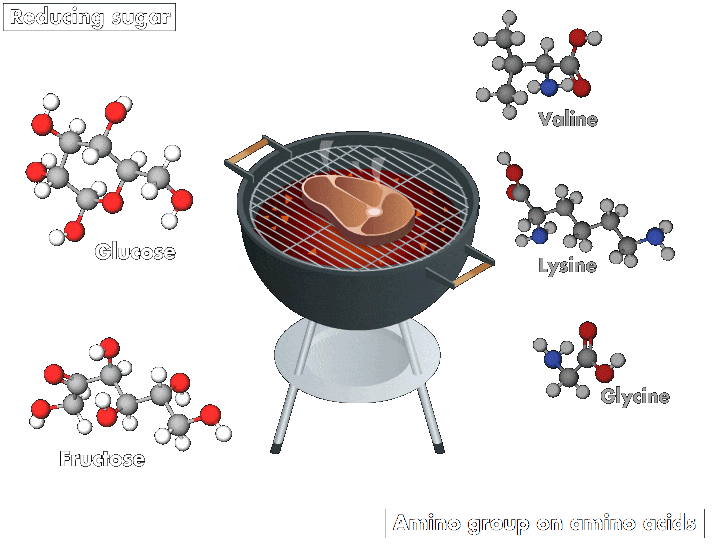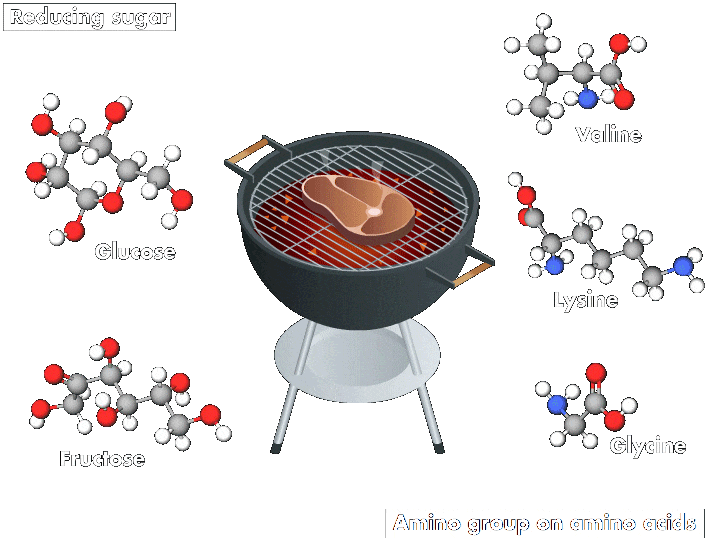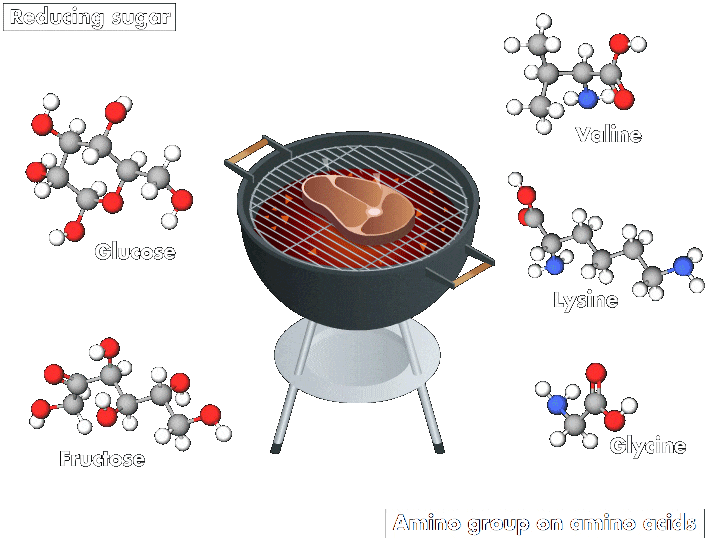
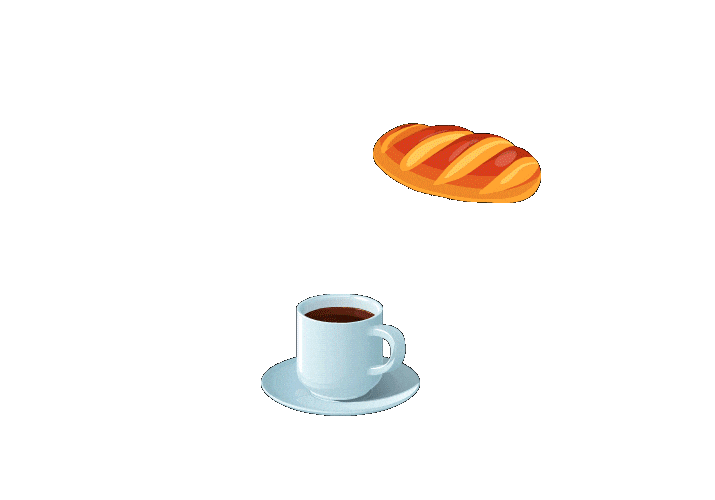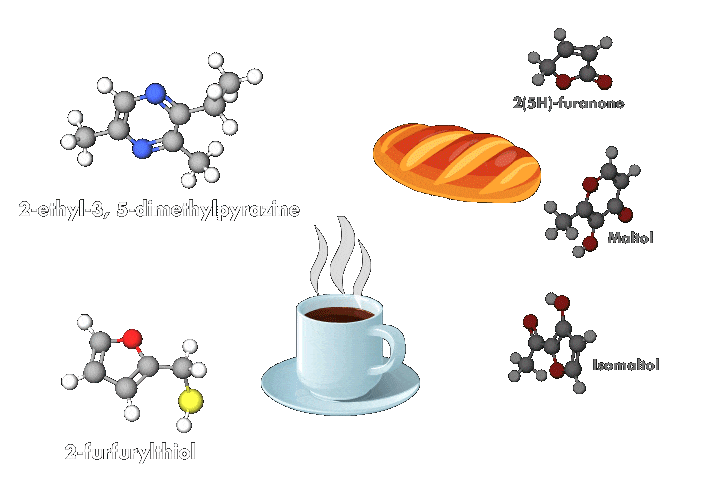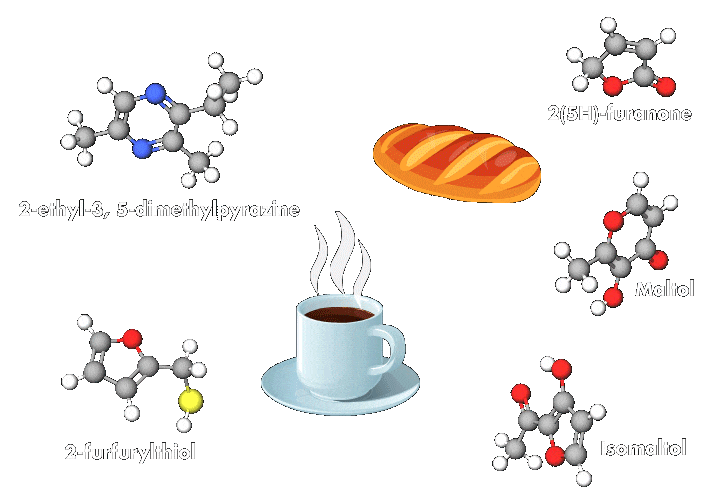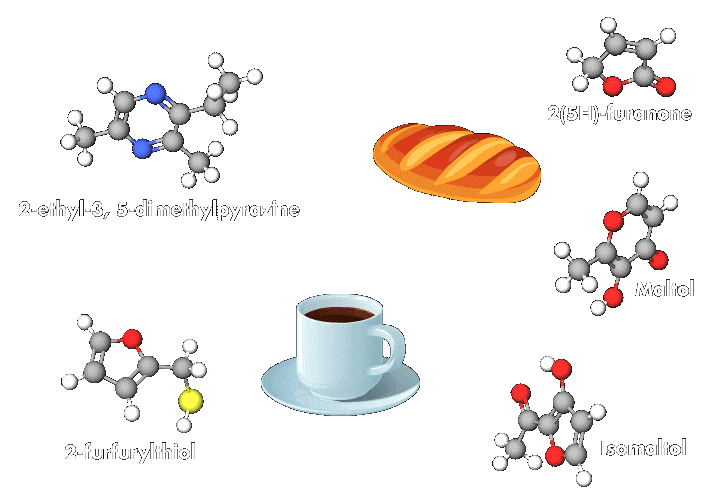
And whether roasting chicken for a biryani or grilling a succulent steak on a barbecue, all lovers of meat and potatoes benefit from a common chemical process: the Maillard reaction, which involves a series of interactions. The reaction starts with an initial reaction between a reducing sugar, such as glucose or fructose, and an amino group. These interactions produce an array of compounds, which may themselves then interact.
“This reaction occurs in any biological system, but its rate increases with temperature. It is responsible for the odor, aromas, and colors of cooked products,” explains Emmanuel Bertrand, a biochemist at Aix-Marseille University. Compounds produced by the reaction make many heated foods taste delicious and create other beloved effects, such as the browning of barbecued meats and roasted potatoes and the richness of coffee.
The Maillard reaction has a special place in the story of humankind, says Varoujan Yaylayan, a food chemistry professor at McGill University. “It is the first chemical reaction performed by humans, 800,000 years ago, just after the discovery of fire,” he explains. The allure of untangling this complicated reaction fuels research on its mechanisms to this day. “There are more than 18 different natural amino acids, thousands of proteins, and more than 60 reducing sugar isomers—the possibilities of their interactions are endless,” he says.
While generally delicious, this reaction can also be damaging, Bertrand notes. When pushed to the limits—overcooking or burning, for instance—the Maillard reaction can reduce the nutritional value of food products by degrading essential amino acids. In charred potatoes, for instance, the reaction can produce carcinogenic compounds, such as acrylamide.
According to Yaylayan, these drawbacks are hot topics in Maillard reaction research. Future advances will likely include the ability to prevent reaction pathways that produce carcinogenic compounds and control those that generate tastes and aromas, he says.
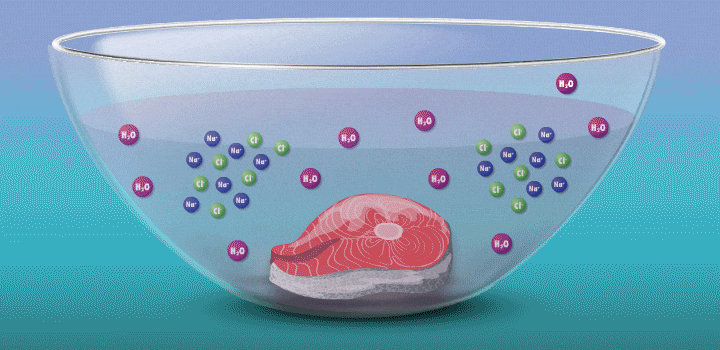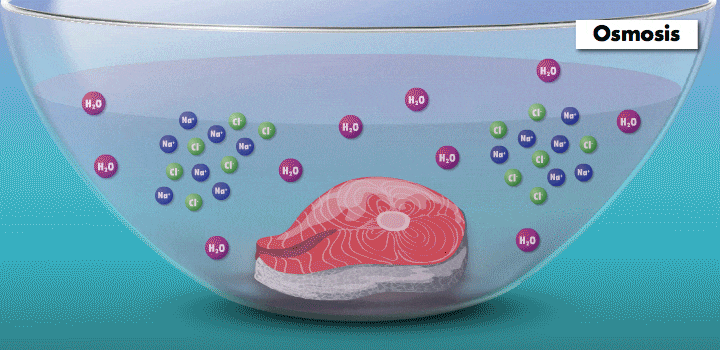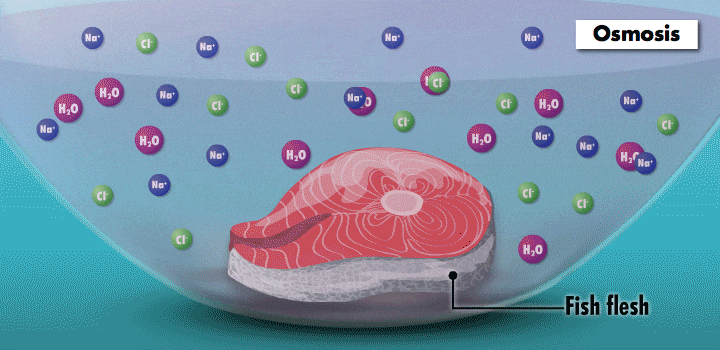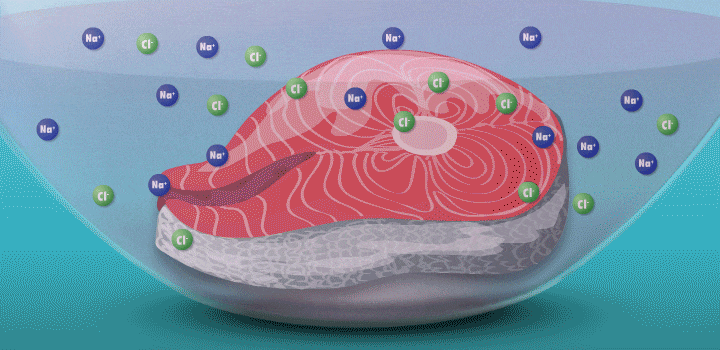
Something fishy
The mouthwatering effects of the Maillard reaction on a nicely browned steak are caused by heat. By contrast, nonthermal “cooking” is responsible for one of Central and South America’s most famous dishes, ceviche.
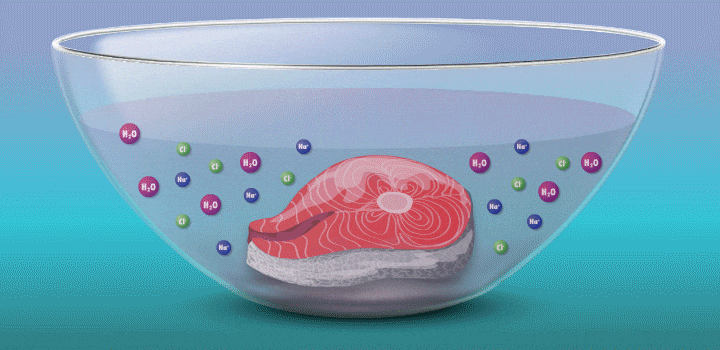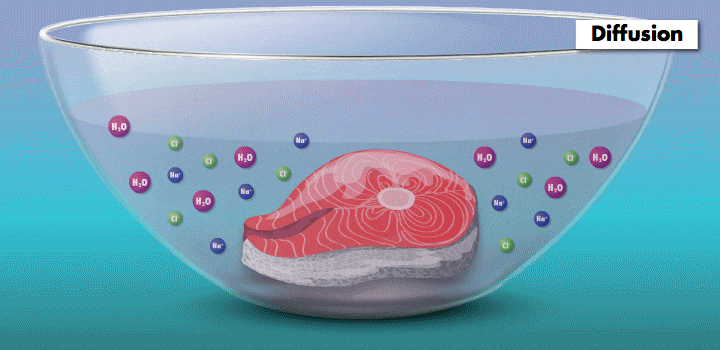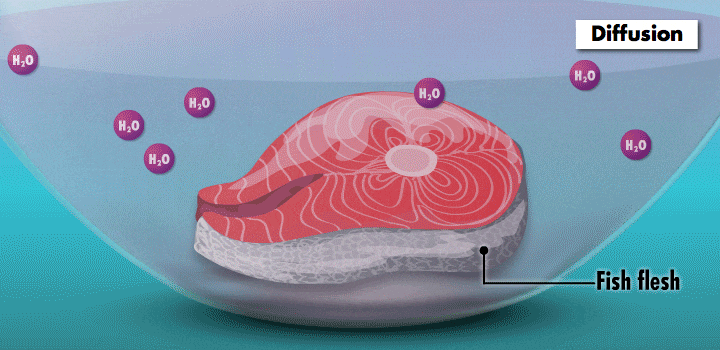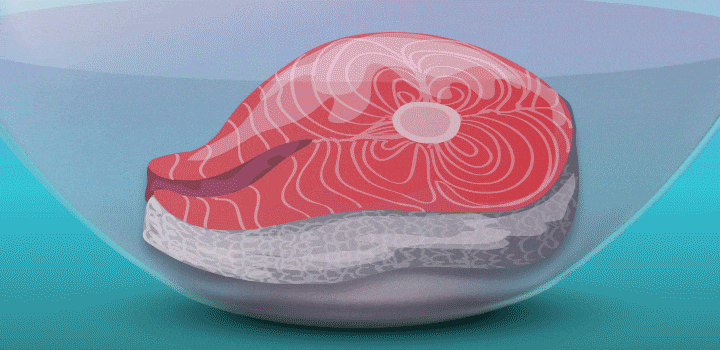
Raw fish can contain toxins or pathogens, and the heat used for cooking can eliminate these hazards. Ceviche—originated over 2,000 years ago in Peru—achieves safer cuisine by a different principle. This classic seafood dish, composed of chunks of seafood, is marinated in citrus juice and mixed with cilantro, onion, and other ingredients. (In its original incarnation, Incan cooks cured fish in chicha, a beverage made from wild fruits or vegetables, such as yellow corn and potatoes.)
During preparation, the fish is chemically “cooked” to make it safe to eat. The acidic lemon or lime juice destroys harmful pathogens because they cannot survive in low pH levels. It also denatures the fish proteins, breaking up amino acid chains in the flesh and rearranging their molecular structure; the meat becomes denser, like cooked fish.
In Scandinavian countries, inhabitants have devised their own distinctive culinary traditions for preparing raw fish. In a region known for harsh winters, pickling and drying have long been used to produce microbial actions that preserve food. Salted, fermented fish dishes, such as Swedish surströmming and Norwegian rakfisk, use techniques that harken back to Viking days. When the fish ferments in its own juices, natural preservatives are released—for example, enzymes in the surströmming Baltic herring produce lactic acid and hydrogen sulfide.
Perhaps most striking is Iceland’s national dish, kæstur hákarl, made from shark that’s fermented by burial in volcanic soil. During fermentation—which lasts several weeks or months—stones and sand are placed on top of the shark, the pressure discharging liquid from its flesh, along with poisons, such as trimethylamine oxide and uric acid. The resultant smell may be pungent, but the taste is delicious—at least according to its Icelandic fans.
Rise and caramelize
Chemistry is perhaps most famously exploited in creating desserts and breads. Chemical processes can be seen in rising dough, caramelizing sugar, flambéing boozy desserts—the list goes on.
Sharma has always had a sweet tooth, and while making the transition from medical researcher to chef, he spent two weeks moonlighting at a patisserie. There he learned scientific culinary techniques, like emulsification, that continue to intrigue him. “The lecithin that’s in egg yolk is fascinating,” he says. “Just to see how it interacts with air, with fats around it. It can actually build what is essentially a mesh when it bakes in the oven.”
Homemade ice cream is a favorite in Sharma’s dessert repertoire. “If you’re using egg yolk, you want the amylase enzyme inside the yolk to break down; that’s why you have to reach a certain temperature,” he explains.

Molecules in eggs give rise to sweet treats.

That Rising Feeling
Batter rises thanks to leavening, a reaction that gives cakes and cookies volume, form, and texture.
Beating eggs into dough introduces air bubbles into the batter. Meanwhile, water in the batter, when heated in the oven, converts to steam. When the air bubbles interact with the steam, the bubbles expand, causing the batter to rise.

Protein-protected air bubbles
In their natural state, egg-protein chains are coiled up and held together by weak peptide bonds. Beating eggs agitates the chains, forcing them to unfold, while the bonds between the chains start to break.
A foam forms as the unfolded proteins interact with each other, trapping air between them. When the uncoiled proteins come in contact with the air, hydrophobic parts of the chains move toward the air and the water in the batter.
As the chains rearrange themselves, new bonds form between them, creating a protein network around the air bubbles. As the network meets heat, it solidifies to protect the air bubbles and keep cakes, cookies, and muffins airy and fluffy.

Whipping up a foam
Whipping egg whites aerates them, producing meringues' characteristic foam. This cloud-like quality is created by the protective network of proteins that form around the air bubbles.
Only egg whites are used in meringue-making, since yolks contain emulsifying fats that inhibit foaming.
Proteins in egg white also prevent meringues and other desserts from collapsing as they leave the oven. As the meringues bake, the proteins coagulate, resulting in a semi-solid web of egg-white protein that keeps its form even when the air bubbles burst.

Let's Stick Together
Fats and water do not mix naturally. Emulsifiers, though, help them bind together—and eggs are excellent emulsifiers. Lecithin is one of the most important emulsifiers found in egg yolk, its hydrophilic ends attracted to water and fastening to fat. Lecithin, therefore, can hold fat and water together.
Thanks to the emulsifying action of eggs, deserts like cake, mousse, and ice cream get their smooth, velvety texture.
But chemistry is a delicate balancing act, so bad timing and incorrect measurements produce unwanted results: Your bread will flop; your meringue will collapse.
This problem is compounded when riding 129 km/hour on train tracks. Just ask Hans Plugge, a self-taught volunteer chef on the private railroad car Dover Harbor. (For full-time work, Plugge is a senior toxicologist at Verisk 3E, a provider of chemical, regulatory, and compliance information services.) Plugge cooks from scratch for passengers on short hops from Washington, D.C., to Williamsburg, Va., as well as on longer trips to places like Chicago and New Orleans. Along with other chefs working to recreate the golden era of railway travel, Plugge also hosts the annual dining extravaganza Chef’s Table, during which eight courses are prepared and served on the railroad car.
“When you’re trying to make desserts especially, there’s definitely chemistry involved in getting things to set,” Plugge explains. This includes altering the amounts of gelling agents used to account for train movement. So, for panna cotta, a molded Italian dessert, “we tend to make them a little stiffer; otherwise, the movement of the car will cause waves,” he says.
A recipe for understanding
Plugge and Sharma share the distinction of being both scientists and masters of the culinary arts. Talking about the parallels between the two, Sharma becomes animated. One of the greatest links, he says, is an inquiring mind. “I’m always curious; I’m always thirsty to understand.”
But there are also more concrete connections. Think of adding salt or sugar as an abrasive when grinding ingredients, says Sharma, recalling how he used magnetic beads to break down cells during medical research. “That was essentially the same thing,” he says. “But those magnetic beads are banging against the cells and rupturing the membrane.”
The blender, too, can work double duty, as both a chef’s appliance and a chemist’s tool. Sharma remembers using a heavy-duty blender to smash apart tissue and extract proteins in the laboratory. Nowadays, he does the same thing in his kitchen, but the results, which include mint-and-garam-masala burgers and peppermint marshmallows, sound far tastier.
CORRECTION: This story was updated on Jan. 14, 2019, to correct the ingredient that turns turmeric red. It is baking soda, not lemon juice, as originally stated in the story.
Precipitating the conversation:
A Perspective from Randal Perry, Technical Fellow at The Chemours Company

Would you like a pungent odor coming from your codfish? How about if it quivered to the touch like jelly—would that entice you? Well, that’s what you’d get if you had some lutefisk, a Nordic delicacy. For those hailing from other parts of the world, it is an acquired taste, as are many other traditionally preserved foods. Before the arrival of refrigeration, traditional preservation methods, such as salting and fermentation, were the only ways to store perishable food.
Nowadays, the fridge in the kitchen, with all its amazing features, works wonders. But consider the power of a much less heralded appliance: the restaurant cooler. It has to work in a much harsher environment, battling limited ventilation, slicks of grease, and high ambient temperatures. Helping restaurant coolers prevail: advanced low global warming potential (GWP) refrigerants like Opteon™, which have low discharge temperatures, reducing the burden on already-stressed refrigeration equipment (and already-stressed line cooks). Keeping expensive ingredients at optimal temperatures helps make restaurant food safe and delicious.
Refrigeration also keeps home cooking wholesome and nourishing. But no matter where people eat, food is perishable—none more so than seafood. And these delicate delicacies need to travel to get to your plate. Luckily, a whole cold chain runs from the fishing trawlers that harvest the catch to the cook who puts the fillet in the skillet. And all of it depends on refrigeration.
No matter if it’s apples reserved for the off-season, unripe bananas waiting their turn in produce, or fine fish on hold for the discerning diner, anything in cold storage must ship dependably and safely. One leak of a toxic refrigerant could ruin millions of dollars’ worth of inventory, as could equipment breakdown brought on by extreme conditions. Fortunately, refrigerants have advanced; ones like Opteon™ are safe and have very low GWP, which makes your sustainably harvested seafood sustainable for the rest of the planet, too.
New refrigerants also enable sustainability when people shop. Online retailing means that nonperishable staple goods are often shipped directly to consumers, leaving food markets free to focus on perishable, healthy food. In fact, as a result, markets are building modular, closed-door refrigerated sections.
Still, somewhere near the array of fresh fish, hills of broccoli crowns, or acres of Greek yogurt, you’ll find a few boxes of salt cod. Old favorites like that can be delicious, but they’re not to everyone’s taste. So if you turn up your nose at traditionally preserved foods like lutefisk, at least modern refrigeration gives you many more options.
READ ABOUT HOW CHEMOURS PRODUCTS ENABLE FOOD TRUCKS AT:
HOW CHEMISTRY ROCKS MUSIC FESTIVALS
PACKING THE RIGHT MOLECULES FOR THE GREAT OUTDOORS
HOW CHEMISTRY THRILLS IN AMUSEMENT PARKS
CHEMISTRY ON ICE
CHEMISTRY TAKES A HOLIDAY
ABOUT SPONSORED CONTENT
Sponsored content is not written by and does not necessarily reflect the views of C&EN's editorial staff. It is authored by writers approved by the C&EN BrandLab and held to C&EN's editorial standards, with the intent of providing valuable information to C&EN readers. This sponsored content feature has been produced with funding support from The Chemours Company.