Advertisement
Grab your lab coat. Let's get started
Welcome!
Welcome!
Create an account below to get 6 C&EN articles per month, receive newsletters and more - all free.
It seems this is your first time logging in online. Please enter the following information to continue.
As an ACS member you automatically get access to this site. All we need is few more details to create your reading experience.
Not you? Sign in with a different account.
Not you? Sign in with a different account.
ERROR 1
ERROR 1
ERROR 2
ERROR 2
ERROR 2
ERROR 2
ERROR 2
Password and Confirm password must match.
If you have an ACS member number, please enter it here so we can link this account to your membership. (optional)
ERROR 2
ACS values your privacy. By submitting your information, you are gaining access to C&EN and subscribing to our weekly newsletter. We use the information you provide to make your reading experience better, and we will never sell your data to third party members.
Process Chemistry
Patrick Fier
Manufacturing magician is creating chemical transformations to scale up drug syntheses
by Bethany Halford
August 20, 2021
| A version of this story appeared in
Volume 99, Issue 30

Credit: Rebecca Green (Fier); Merck & Co. (pills); Shutterstock (flask)
When Patrick Fier finished high school, he didn’t know what he wanted to study, so he started taking classes at community college. Two years later, he transferred to the University of Northern Iowa, where he thought he’d take the necessary courses for applying to medical school. But Fier’s new adviser discouraged him from taking organic chemistry, warning that it was too challenging for transfer students.
Fier decided to take organic chemistry anyway. To get a head start, he began reading the textbook a few days before the first class. Within 2 weeks, he had devoured Peter Vollhardt and Neil Schore’s 1,200-page text Organic Chemistry: Structure and Function. “Once I got into the reactions, something just sparked in me,” Fier recalls. “Prior to that, I would certainly have never considered a career in chemistry. And then, after that semester, I switched my major to chemistry.”
Advertisement
More than a dozen years later, Fier’s love of practical organic chemistry and deep dives into reaction mechanisms are evident in his work as a process chemist at Merck & Co. He develops large-scale reactions to make the company’s drugs and drug candidates and develops new chemical reactions when he can find a spare hour or two. “I love doing chemistry and being involved with the nitty-gritty chemistry details,” he says.
“Patrick Fier’s ability to identify the intersection between what’s important and what’s achievable is uncanny,” says Fier’s doctoral mentor, John Hartwig, a chemistry professor at the University of California, Berkeley. “More chemistry is invented by him on Friday afternoons than by most people all week.”
In 2020, Fier led the effort to make molnupiravir, an experimental SARS-CoV-2 antiviral, on large scale. It was a high-pressure project that began in early June 2020 as COVID-19 spread around the world. Fier and his team needed to make enough molnupiravir for a clinical trial. When a company launches a new drug, the process chemistry team is typically tasked with making about 100 kg of the active pharmaceutical ingredient, sometimes less if it’s a particularly potent cancer chemotherapy, Fier says. But Merck wanted a route that could produce 500,000 kg of molnupiravir annually.
Fier helmed two simultaneous efforts for making the molecule. One route boosted the overall yield of the 10-step synthesis from 37% to 58%. “It’s not elegant chemistry in the classical sense,” Fier says, but it gets the job done. It has now been used to make more than 35,000 kg of molnupiravir.
The second route is faster and cheaper but took longer to develop. The three-step synthesis features enzyme-catalyzed reactions that wed ribose and uracil, which are commodity chemicals. Fier’s team had developed the route by October 2020, and by January, the scientists were able to perform the chemistry on 100 kg scale.
Fier says his work on molnupiravir required the efforts of a large team of scientists. But he also works in much smaller groups or on the occasional solo effort to develop interesting chemical transformations. For example, together with his Merck colleague Kevin Maloney, Fier developed a way to make phenols—common motifs in many drugs and drug-like molecules—from haloarenes using inexpensive, air-stable reagents. The reaction has been used more than 800 times at Merck alone on both small scale for drug discovery and on kilogram scale to make drug candidates for clinical trials.
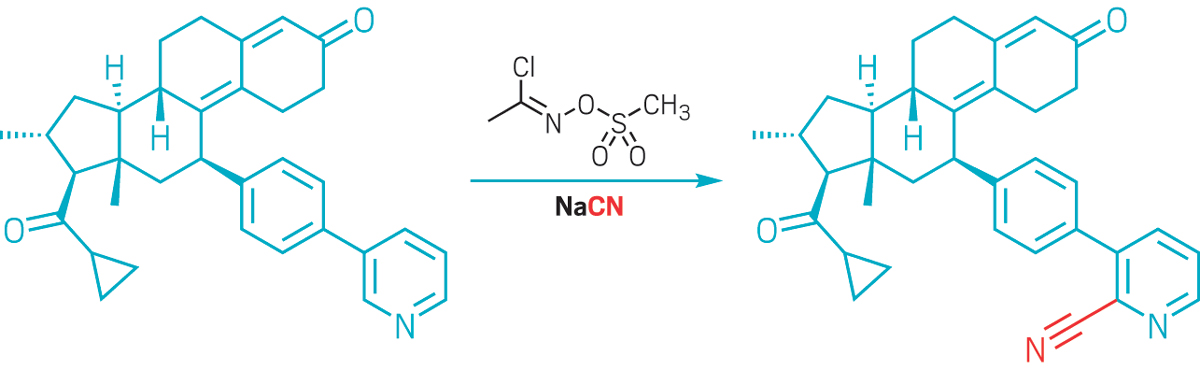
Fier has also been working on multifunctional reagents, single-molecule Swiss army knives that can be used for doing multiple transformations on a single substrate. One such reagent contains a nucleophile, an activating group, and an oxidant, all of which work in concert to tack amines on to pyridines.
When he’s not working at the bench, Fier builds furniture. It’s just part of his nature to build practical things, he says, whether it’s an end table or a useful molecular motif.
Vitals
Current affiliation: Merck & Co.
Age: 34
PhD alma mater: University of California, Berkeley
Hometown: Bettendorf, Iowa
If I were an element, I’d be: Sodium. “Naturally, I’m in a ‘positive’ mood.”
Role model: R. B. Woodward. “He was an incredibly creative problem solver, developed remarkably elegant syntheses with a limited toolbox of reactions, and had immeasurable impacts on the field of organic chemistry.”
When Patrick Fier finished high school, he didn’t know what he wanted to study, so he started taking classes at community college. Two years later, he transferred to the University of Northern Iowa, where he thought he’d take the necessary courses for applying to medical school. But Fier’s new adviser discouraged him from taking organic chemistry, warning that it was too challenging for transfer students.
Fier decided to take organic chemistry anyway. To get a head start, he began reading the textbook a few days before the first class. Within 2 weeks, he had devoured Peter Vollhardt and Neil Schore’s 1,200-page text Organic Chemistry: Structure and Function. “Once I got into the reactions, something just sparked in me,” Fier recalls. “Prior to that, I would certainly have never considered a career in chemistry. And then, after that semester, I switched my major to chemistry.”
More than a dozen years later, Fier’s love of practical organic chemistry and deep dives into reaction mechanisms are evident in his work as a process chemist at Merck & Co. He develops large-scale reactions to make the company’s drugs and drug candidates and develops new chemical reactions when he can find a spare hour or two. “I love doing chemistry and being involved with the nitty-gritty chemistry details,” he says.
Vitals
▸ Current affiliation: Merck & Co.
▸ Age: 34
▸ PhD alma mater: University of California, Berkeley
▸ Hometown: Bettendorf, Iowa
▸ If I were an element, I’d be: Sodium. “Naturally, I’m in a ‘positive’ mood.”
▸ Role model: R. B. Woodward. “He was an incredibly creative problem solver, developed remarkably elegant syntheses with a limited toolbox of reactions, and had immeasurable impacts on the field of organic chemistry.”
“Patrick Fier’s ability to identify the intersection between what’s important and what’s achievable is uncanny,” says Fier’s doctoral mentor, John Hartwig, a chemistry professor at the University of California, Berkeley. “More chemistry is invented by him on Friday afternoons than by most people all week.”
In 2020, Fier led the effort to make molnupiravir, an experimental SARS-CoV-2 antiviral, on large scale. It was a high-pressure project that began in early June 2020 as COVID-19 spread around the world. Fier and his team needed to make enough molnupiravir for a clinical trial. When a company launches a new drug, the process chemistry team is typically tasked with making about 100 kg of the active pharmaceutical ingredient, sometimes less if it’s a particularly potent cancer chemotherapy, Fier says. But Merck wanted a route that could produce 500,000 kg of molnupiravir annually.
Fier helmed two simultaneous efforts for making the molecule. One route boosted the overall yield of the 10-step synthesis from 37% to 58%. “It’s not elegant chemistry in the classical sense,” Fier says, but it gets the job done. It has now been used to make more than 35,000 kg of molnupiravir.
The second route is faster and cheaper but took longer to develop. The three-step synthesis features enzyme-catalyzed reactions that wed ribose and uracil, which are commodity chemicals. Fier’s team had developed the route by October 2020, and by January, the scientists were able to perform the chemistry on 100 kg scale.

Fier says his work on molnupiravir required the efforts of a large team of scientists. But he also works in much smaller groups or on the occasional solo effort to develop interesting chemical transformations. For example, together with his Merck colleague Kevin Maloney, Fier developed a way to make phenols—common motifs in many drugs and drug-like molecules—from haloarenes using inexpensive, air-stable reagents. The reaction has been used more than 800 times at Merck alone on both small scale for drug discovery and on kilogram scale to make drug candidates for clinical trials.
Fier has also been working on multifunctional reagents, single-molecule Swiss army knives that can be used for doing multiple transformations on a single substrate. One such reagent contains a nucleophile, an activating group, and an oxidant, all of which work in concert to tack amines on to pyridines.
When he’s not working at the bench, Fier builds furniture. It’s just part of his nature to build practical things, he says, whether it’s an end table or a useful molecular motif.

















Join the conversation
Contact the reporter
Submit a Letter to the Editor for publication
Engage with us on Twitter